Our Food Is Radioactive!!
Hello fellow scientists and science enthusiasts !
Today, we will see whether it is necessary to witness a nuclear accident up-close to get a dose of radioactive radiation, or is it that we are always exposed to radiation without even being aware of the fact!!!


Types of radiations:
- Ionizing radiation : Any radiation that is sufficiently powerful to ea able to rip apart electrons from the atoms, thus ionizing them. It can be composed of charges particles, electrons or atoms moving at high speed of approximately 1% that of light. High energy light waves are also a part of this. Such a radiation is generally the result of radioactive decay. It can cause damage to living tissues, cells and the DNA.
- Non-ionizing radiation : It is quite the opposite of the former. This includes microwaves, radio waves etc. Radiation released from mobile sets fall in this category. It has non-radioactive origins. This doesn't cause any significant damage to life.
Note : Throughout the rest of the article, the term "radiation" will be used interchangeably with and will mean the same as "ionizing radiation".
Don't know what is Radioactivity?
Some atoms are stable, others are not. Those which are unstable try to attain stability by removing some of there constituents causing the instability. Thus an unstable atom emits either particles or electromagnetic radiation and decays to a more stable structure. Depending upon the type of emission, the radiation can be classified as:- Alpha rays -These are basically streams of Alpha particles (He++particles). These are comparetively large particles, and are not very energetic. Cannot penetrate much.
-
Beta rays - These are streams of electrons.
- Gamma rays - These are electromagnetic waves (like light, but are highly energetic and invisible to the naked eye).
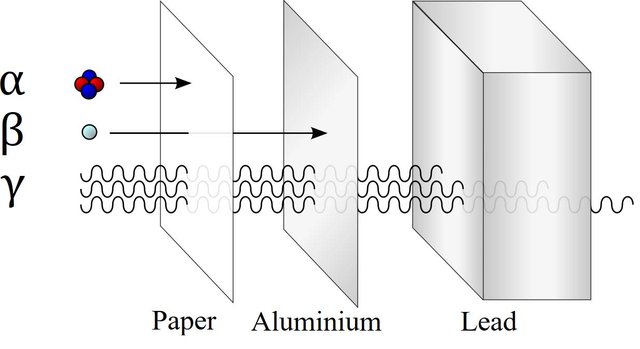
Comparison of penetration power
[Source : [WikiMedia Commons](), Author : https://commons.wikimedia.org/wiki/User:Stannered, License : https://creativecommons.org/licenses/by/2.5/deed.en]
Why some materials attain radioactivity?
Atoms are the basic building blocks of all matter in the universe. Atoms are further made up of sub-atomic particles called : electrons(-), protons(+) and neutrons(0). The protons and neutrons make up a central dense structure called the nucleus. The electrons revolve around the nucleus.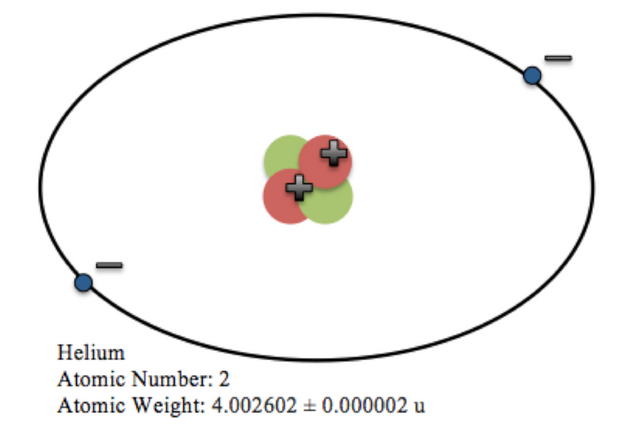
Cartoon of Helium atom.
[Source : [WikiMediaCommons]( ), Author : By Rileyfricke - Own work, CC0, https://commons.wikimedia.org/w/index.php?curid=39059083]
), Author : By Rileyfricke - Own work, CC0, https://commons.wikimedia.org/w/index.php?curid=39059083]
- Attractive Forces : These forces bring particles closer together, thus reducing the energy of the system, and making it more stable.
- Repulsive Forces : Such forces generally contribute to instability of the system, as they increase the energy of the system in question.
Stability of an atom decides whether or not it will be radioactive. This in-turn is determined by the ratio of neutrons to protons in an atom. The ratio of the number of neutrons to the number of protons in a stable atom is either greater than or equal to 1.
Now, if we think of protons as imaginary magnetic monopoles, then the neutrons can be thought of as being pieces of magnetic metal. So, while the monopoles will repel each other due to similar charge, the metal pieces and hence the neutrons help in bringing stability by reducing the proton-proton repulsions. Now, for smaller atoms until around Calcium(Z=20), the number of neutrons in the nucleus remains the same as the number of protons, and hence the N/Z ratio remains equal to unity. But, as the atomic number increases further, the number of neutrons required to maintain stability of the atom increases, hence the n/p ratio becomes >1, and may reach uptill a value of 1.54This can be easily visualized by the following plot of protons vs neutrons of stable nuclei :
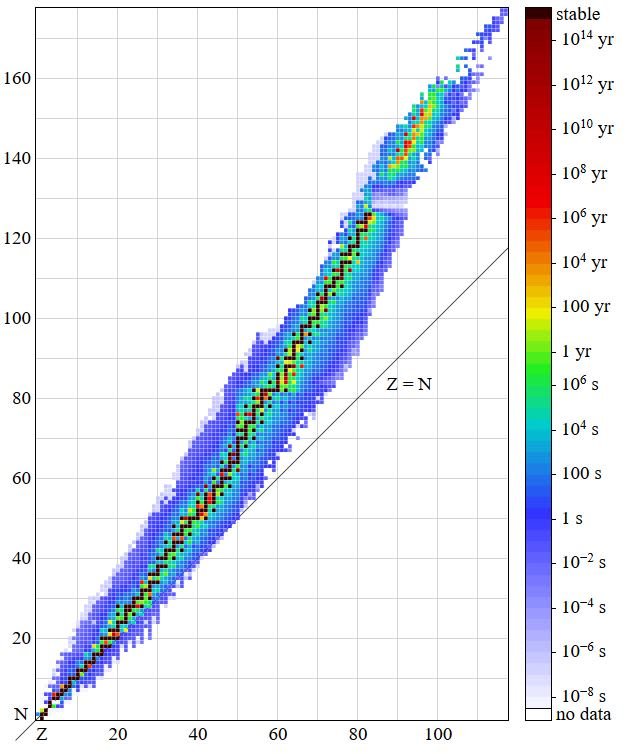
 ), Author : By BenRG - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=7900237 ]
), Author : By BenRG - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=7900237 ]Measuring Radioactivity
The figure below gives a very simple and accurate representation of the whole process of radioactive decay, its rate measurement, propagation, and measurement of its effect on objects.
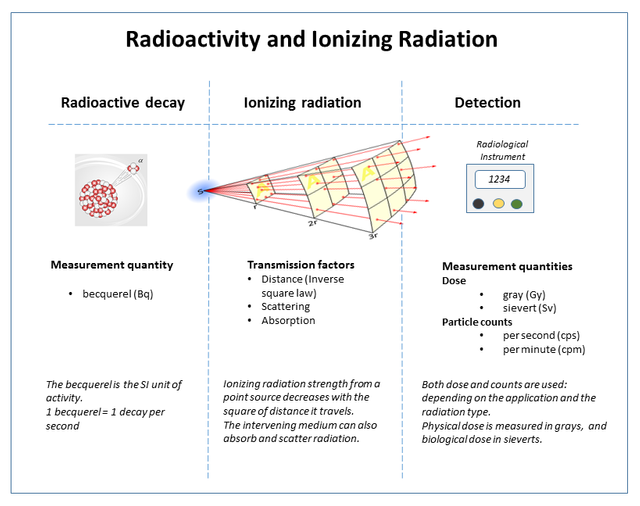
 ), Author : By Doug Sim - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=33608316 ]
), Author : By Doug Sim - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=33608316 ]So, there are a number of ways and stages at which ionizing radiation can measured : as the number of atoms undergoing decay per unit time in a given sample, as particle counts (particles detected per unit time at a particular location in space), as radiation dose (this quantifies the effect the levels of ionizing radiation at a particular place can have on humans/life in general).
Depending upon the use, different units are used. What we are interested in this article is the radiation dose.
Units for measuring radiation dose :
- Sievert (Sv) = 1 joule/kilogram – a biological effect. The sievert represents the equivalent biological effect of the deposit of a joule of radiation energy in a kilogram of human tissue. The equivalence to absorbed dose is denoted by Q.
- Gray (Gy) = 1 joule/kilogram – a physical quantity. 1 Gy is the deposit of a joule of radiation energy per kg of matter or tissue.
Radiation Exposure Levels
Here is an interesting comparison of various radiation doses published by the Oregon State University :
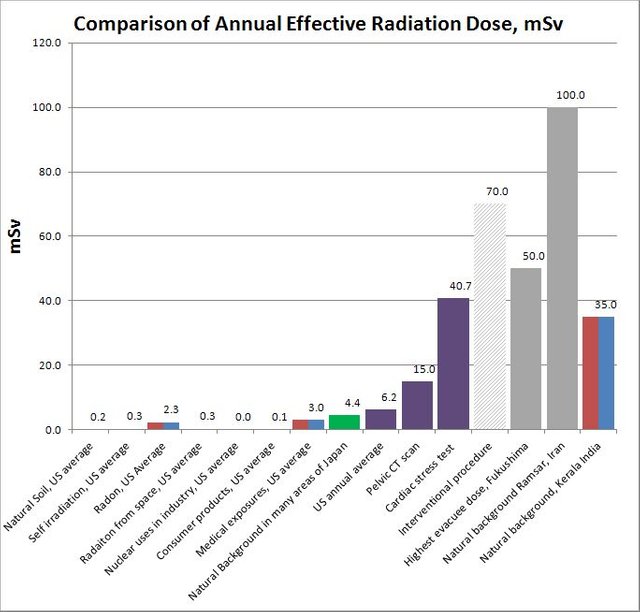
Radiation dose comparison
[Source : Flickr, Author : [Oregon State University](https://www.flickr.com/photos/oregonstateuniversity/), License : [CC by 2.0](https://creativecommons.org/licenses/by-sa/2.0/)]
Natural Isotopes - the cause of "natural" radioactivity
Some elements occur naturally in more than one isotopic forms or simply isotopes. The isotopes of a particular element differ only in the number of neutrons in the nucleus. Hence, while some isotopes of an element can be stable, others can be radioactive. Every element is found naturally as a mixture of its isotopes in certain ratios. Isotopes of an element are chemically identical and give same reactions with other substances.
For example : Naturally occurring potassium is composed of three isotopes, of which 40
K is radioactive. Traces of 40
K are found in all potassium, and it is the most common radioisotope in the human body.
So, whenever a plant absorbs an element from the soil, it absorbs all the isotopes of the element naturally present in the soil. A plant cannot differentiate between radioactive and non-radioactive isotopes of an element as they are chemically the same.
Potassium Trouble
Potassium is probably the most abundant mineral in the soil and is required by plants and animals in significant quantities.
We have already seen that an isotope of potassium, K-40 is radioactive. This and other radioactive isotopes of other elements impart a significant measurable amount of radioactivity to plant parts - fruits, leaves etc.
A very popular example is that of banana.
CONCLUSION
There is no reason to be afraid. The amounts of radiation doses we receive from the outer space is most of the times equal to or higher than the doses we are talking of. So, there is nothing we can possibly do about it. This is natural.
Interesting Fact : Do you know the amount of radiation exposure required to kill you instantly? Well that is about an exposure of around 2 Sv or more all at an instant. So, comparing that with the exposure from a banana (0.1 micro Sv), you can easily tell, the banana hardly has any power!!
More over, our body has evolved mechanisms to rectify the small damages in DNA caused by these radiations.
SO NO NEED TO WORRY!
Keep Steeming, Keep Learning
Stay informed...
M.Medro
References :
- Definition of Ionizing Radiation : https://en.wikipedia.org/wiki/Ionizing_radiation
- The Most Radioactive Places on Earth : https://www.youtube.com/watch?v=TRL7o2kPqw0
- World Health Organisation - Ionizing Radiation : https://www.who.int/ionizing_radiation/about/what_is_ir/en/
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io and @curie.
If you appreciate the work we are doing then consider voting all three projects for witness by selecting stem.witness, utopian-io and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for the comprehensive review. In passing, I really dislike the magnetic monopole analogy, because such a thing does not exist. it is thus very hard to imagine something that does not exist...
It is preferable to say that there are two forces fighting with each other inside a nucleus: a strong force that is binding the nucleons together and that is short range, and an electric repulsion between the protons that is long range. These two forces behave thus differently depending on the distance between the interacting objects.
In a few words, more neutrons are needed in large nuclei so that we have enough strongly interacting pairs to prevent the electric force from breaking the nucleus apart.
Hmm....that's a great reply indeed.
Thanks a lot @lemouth for your valuable insights on the topic.
I'll definitely try to modify and replace that analogy with something better...
Thank you so much.
Regards
M.Medro
You are welcome! :)
Congratulations @medro-martin! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Do not miss the last post from @steemitboard:
Hi @medro-martin!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Interesting! So, is there any food that, due to its composition or the condition in which it grows or is produced, is more radioactive than the rest? Just wondering
Yes you are right. That's exactly the case.
So, though giving an example is a trivial thing to do, but generally speaking, if you remove all the radioisotopes of all the major elements from the soil while leaving the stable ones, the plant will have no choice but to absorb those present, and hence it will be less radioactive.
Thanks
M.Medro