What Is The Smallest Fragment Of Time? Scientists Have Measured...
Zeptosecond: one trillionth of a billionth of a second!
This amazing discovery was made by the team members of Max Planck Institute for Quantum Optics in Garching, Germany. The team members were studying Albert Einstein's photoelectric effect when they made this amazing discovery of the smallest fragment of time, Zeptosecond. Yes, scientists have measured the smallest fragment of time. Earlier it was the time required for light to travel the Planck length. The speed of light in a vacuum is about 300,000,000 m/s and the Planck length is about 1.616E-35 m, so on behalf of these two values, the smallest fragment of time would be 5.28E-44 seconds.
This value is also known as Plank time
This value is also known as Plank time and this is the shortest time division possible. Earlier the smallest measured time was 100 attoseconds. But now, scientists have measured smallest fragment of time even smaller than the Plank time.
After so many effort and sleepless nights
 As far we have understood time, time is quantized same as matter and energy. For us, a single second matters a lot but the world around us and the scientists have gone more precise in the measurement of time. After so much effort and sleepless nights, the great Physicists were successful in measuring the changes in an atom on the level of zeptoseconds. Zeptosecond is now the smallest fragment of time which is a trillionth of a billionth of a second, this is the smallest fragment of time ever measured in the history of science.
As far we have understood time, time is quantized same as matter and energy. For us, a single second matters a lot but the world around us and the scientists have gone more precise in the measurement of time. After so much effort and sleepless nights, the great Physicists were successful in measuring the changes in an atom on the level of zeptoseconds. Zeptosecond is now the smallest fragment of time which is a trillionth of a billionth of a second, this is the smallest fragment of time ever measured in the history of science.
it is also known as Photomission
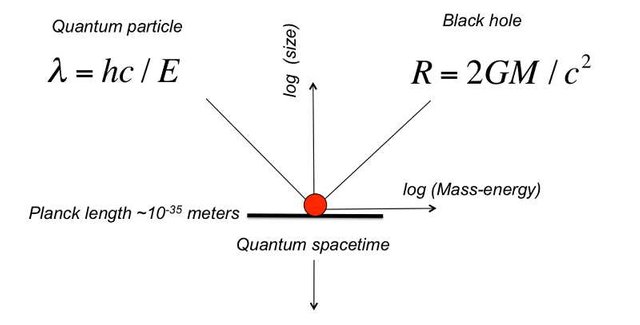
The photoelectric effect was given by Albert Einstein in the year 1905.This process is defined as the production of electrons or you can say production of free carriers when light falls on a material. The electrons which are emitted in this process are known as Photoelectrons.
In Einstein's photoelectric effect
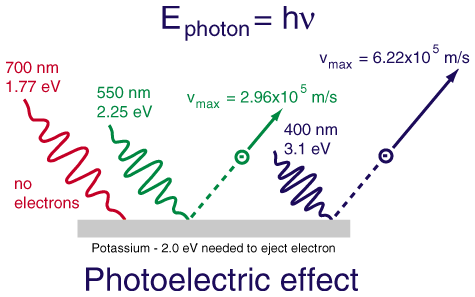
In other words, the photoelectric effect occurs when photons, also known as particles of light, strike the electrons orbiting an atom.If we look at the quantum mechanics theory, then we will see that the energy from these particles of light is either absorbed by a single electron or divided among few electrons. But till now, no was able to judge how it is decided, no one has been able to study this energy division process.
The value lies between 5 to 15 attoseconds
The final result is an electron going from the bonds of its parent atom. In the previous researches, it has been shown that the whole process from the starting to the end takes around 10 attoseconds. It is an average as the value lies between 5 to 15 attoseconds. An attosecond is 10-18 seconds.Before this, in all the researches scientists were only able to measure in detail that what happened after the electron leaves the bond of parent atom.
Max Planck Institute of Quantum Optics in Germany
But the team members of Max Planck Institute of Quantum Optics in Germany were able to see the process from the other side and found out that what exactly happens in that tiny little amount of time before the electron leaves the bond of its parent atom.Now, with this new level and new fragment of time, the scientists were able to measure the whole process of photoelectric effect in which electron is escaping its atom for the first timeSPONSORED
Zeptosecond it is smaller than attosecond

They were able to achieve this by firing a range of lasers at a helium atom, and this helped them out in measuring the entire process of photoelectric effect with the smallest fragment named Zeptosecond it is smaller than an attosecond. Zeptosecond is 10-21 seconds. It is the smallest precision ever measured.Information is given by one of the researchers Marcus Ossiander at New Scientist; he told that "By the help of this information, we can measure the time which the electron took to change its quantum state from the very narrow and tightened bound state around the atom to the Free State."
And they were able to spot some pattern in the results.
The team of scientists who were studying the photoelectric effect and make this new discovery choose helium atoms for their study because helium atom has only two electrons, which means that they are very complex and this will help researchers to measure out the quantum mechanical behavior of the helium atom. They can study how the division of energy takes place between the atoms which was a mystery. And they were able to spot some pattern in the results.
The extremely ultraviolet laser pulse lasted just 100 to 200 attoseconds

Firstly, the team fired a super shot extremely ultraviolet laser pulse at a helium atom in order to excite the two highly bonded electrons. This was under the first set of experiments.The extremely ultraviolet laser pulse lasted just 100 to 200 attoseconds.After making a whole lot of readings across that whole time and side by side calculating their statistical spread, the team members were able to compress down to a total time frame of 850 zeptoseconds.
The first set of experiment
After performing the first set of experiment, they used a near-infrared laser pulse. The infrared laser pulse lasted for four femtoseconds. A femtosecond is 10-15 seconds. Finally, they calculated that the ejection of an electron took around 6 to 20 attoseconds. It totally depends on the interaction of the electron with a nucleus and other electrons.This all makes out the conclusion on how the electrons divide the laser's energy. It was observed that the energy was split evenly between the two electrons and sometimes the division was uneven, and sometimes a single electron took all of the energy alone.There are some factors that affect this division of energy, one of them are the correlation between the electrons and also the electromagnetic state of the laser field which is used by the scientists. Still, there is much more stuff to do. There is a lot of work which is yet to be done, but this step is quite exciting towards the understanding of the mysterious quantum behavior of atoms. This also helps in the study of how electrons work on if they were observed on an individual basis. This whole research was published in Nature Physics.
Do you believe in the theory of the Zeptosecond's existence?
This blogpost for information only...
creadit wittyfeed.com
Congratulations @veeru! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPgood post
Bro follow me i follow u back and upvote