Why is the road sticking?
White sugar and salt are very easy to confuse and you must fall into the scene of your grill "salty" because the "twins" look quite similar.
Conspiracy in the pancakes and the "misery of the century" of fat

But when they add a little water, the twins become completely different. Both salt and sugar crystals begin to dissolve in water, but the sugar is sticky and the salt does not. Why so?
According to How Stuff Works , hydrogen is the key to tackiness. Sugar is a solid, its molecules are made of carbon, hydrogen and oxygen. Sugar crystals are intact and do not stick together - you can easily refine sugar. But with the presence of a liquid, strong oxy-hydrogen bonds in the past will begin to break down and liquid hydrogen atoms will look for something else to stick to.
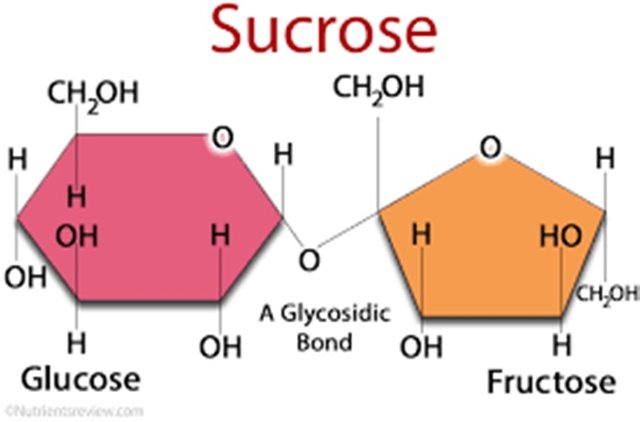
Some hydrogen atoms will stick to the nearest surface, some will pick up hydrogen molecules in the liquid and some will bind to another hydrogen or oxygen atom in the path. As a result, it produces a sticky solution.
Meanwhile, the salt is made of sodium and chlorine, so when it dissolves in water there is no hydrogen floating around to stick to anything.
But what about water? Its molecules are also made up in part by hydrogen - why it does not become sticky like sugar when combined with some other substance? This involves making the structure more complicated than water. A sugar molecule contains 12 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms - and more hydrogen bonds than one water molecule. When the links in the pathway are broken, there will be more opportunities for the molecules to bind to anything they come in contact with, including other sugar molecules.
On the other hand, each water molecule consists of only two hydrogen atoms and one oxygen atom, so it does not have many "sticky points". Therefore, the soluble sugar will be sticky and the water itself is not.
☺️
Done!!! upvote and follow me back
https://steemit.com/joke/@xaqib/joke-no-4
GreaT
done upvote back
gazab
UPVOTE MY CMNT AND FOLLOW BACK