Cyanide detection in aqueous medium with novel Azo dyes

Cyanide
Cyanide is used to refer to chemical compounds having the functional group containing carbon atom attached by triple bond to a nitrogen atom [1, 2]

In Ionic form it is written as CN-
They are found naturally in food, plants, as well as being produced by certain microorganism. Common sources such as car exhaust, cyanide fishing, smoking, organic chemical industries, almonds, apples and cassava are major exposure to cyanide. [3]
Cyanide is toxic to human health and environment. Basic symptoms such as headache, weakness, nausea, seizures and cardiac arrest are reported [4]. The permissible level of cyanide is 0.2 ppm in drinking water. [5]
Adegoke et al reported a facile way of detecting and quantifying cyanide using novel azo dyes [6]. These azo dyes were synthesized and reported [7]. We used these azo dyes as sensor in determining the presence of cyanide in aqueous medium.
Azo dyes are compounds that have functional group of -N=N-. They are basically coloured.
We used the following azo dyes here as a sensing material to detect the presence of cyanide. When cyanide ion reacts with the azo dyes, it changes the initial colour of the azo dyes to a different one. The process is referred to as Colorimeteric sensing and the azo dyes are Colorimetric sensors.
Used Azo Dyes
The following mono azo dyes were used. The name is 4-carboxyl-2, 6-dinitrophenylazohydroxynaphthalenes dyes
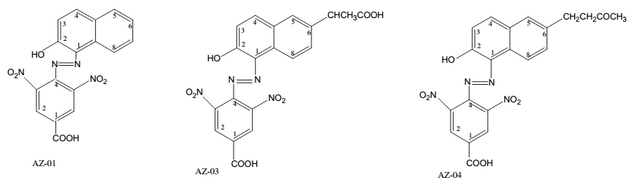
[6]
A UV-VIS spectrophotometer was then used to record the colour changes associated with the reaction of cyanide ion with the azo dyes.
Preparation method
Potassium cyanide was used as the source of the CN ion, the Azo dyes were dissolved in DMSO to make a solution thereof. Thereafter, a certain volume of CN- solution was added to an aliquot of the dyes. The mixture was made up with acetone. The resulting solution was then measured using the UV-vis spectrophotometer. This is because the resulting solution can be easily measured in the visible region of the electromagnetic spectrum.
Temperature and time were optimized to determine the best conditions for the determination. The ratio of reaction between the cyanide ion and azo dyes was done using Job’s method. Interefence studies from several anions, was also investigated.
Findings
We found that upon addition of cyanide to the dyes, different colour ranging from yellow to brownish red developed. The final colour observed varied between purple to lilac based on the azo dyes used.
This consequently makes the spectrophotometer an easy tool to use to determine the contribution from the cyanide ion
The authors showed a typical spectrum recorded for the azo-dyes.
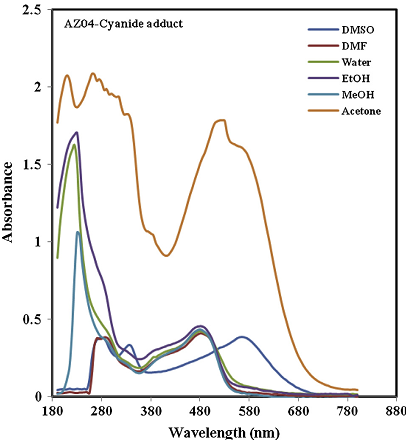
[6]
Potassium Cyanide solution is colourless, therefore, it will have no absorption peak in the visible region. It was obvious from the figure above that the addition of acetone to the mixture of cyanide ion and the azo dyes will give a better characterization.
It was observed that when CN ion was added to the dye labelled AZ-01, the wavelength shifted from 430 nm to 520 nm, having a bathochromic shift of 110 nm. Also, the other two dyes followed the same trend, with maximum wavelength at 525 nm for AZ-03 and 520 nm for AZ-04.
We found that the chemical property responsible for this changes in the azo dye was the nucleophilic character of the CN- ion, as well as the predominance of hydrazine tautomer over the azo tautomer for the dyes, which makes their carbon centre susceptible to CN- nucleophilic attack. This then caused a shifting of the wavelength to more visible region.
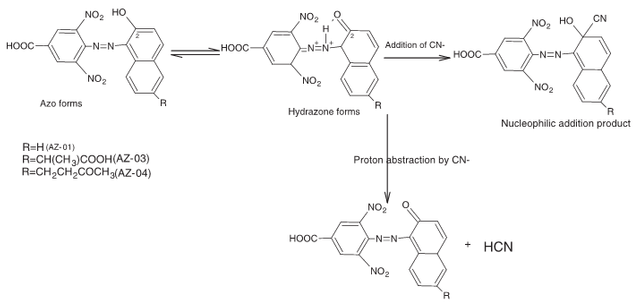
[6]
The optimal temperature for the analysis and detection of the cyanide ion was 30 oC, this is because the highest optimum absorbance was recorded at this temperature. After establishing a suitable temperature, the time to achieve complete reaction was 5 minutes. These two parameters were then used to determine the stoichiometric ratio.
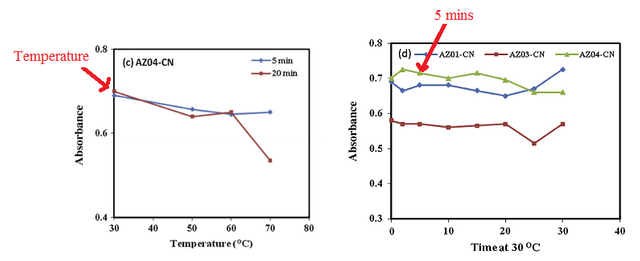
[6]
The ratio at which the CN- reacts with the azo dyes was established using the Job’s plot. It was found that the different azo had different combining ratio with the cyanide ion based on their chemical structures.
The detection limit and quantitation limit studies were performed and summarized in the table below
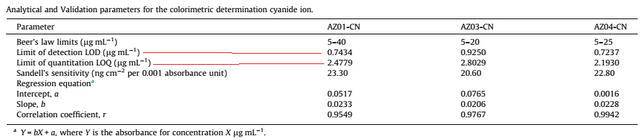
[6]
The limit of detection was different for each azo dye, this is because they have different chemical structures. The limit of detection ranged from 0.7237 – 0.9250 µg ml-1. While the limit of quantitation was from 2.1930 to 2.4779 µg ml-1.
The interference studies revealed that the presence of other anions did not affect the detection of cyanide ion.
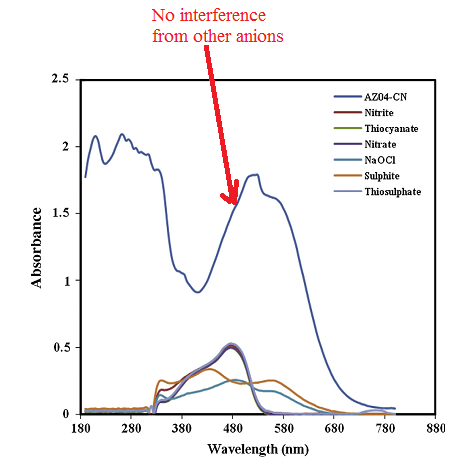
[6]
Recovery studies were in the range of 99.95 to 103.42 %. These show that the synthesized dyes are able to accurately detect cyanide ion in aqueous medium.[6]
Conclusion
The azo dyes here are reproducible and easy to synthesize. The application as a colour changing compound in the presence of cyanide ion makes it easily adaptable to many situations and sources of cyanide while making use of easily available and less costly equipment.
[1]. https://www.cyanideinsight.com/cyanide-poisoning/where-cyanide-is-found
[2]. https://en.wikipedia.org/wiki/Cyanide
[3]. Hijji, Y. M., Barare, B., & Zhang, Y. (2012). Lawsone (2-hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sensors and Actuators B: Chemical, 169, 106-112.
[4]. https://www.health.ny.gov/environmental/emergency/chemical_terrorism/cyanide_general.htm
[5]. ATSDR (Agency for Toxic Substances and Disease Registry), Toxicological Profile for Cyanide (Update). Atlanta, GA U.S. Department of Health and Human Services, Public Health Service, 2006.
[6]. Adegoke, O. A., Adesuji, T. E., & Thomas, O. E. (2014). Novel colorimetric sensors for cyanide based on azo-hydrazone tautomeric skeletons. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 128, 147-152.
[7]. Adegoke O. A, Idowu, S.O., & Olaniyi A.A (2008). Synthesis and spectroscopic characterization of 4-carboxyl-2,6-dinitrophenylazohydroxynaphthalenes. Dyes and Pigments 77, 111 -117.
This is a very interesting and detailed one @turpsy. Reminds me of my old chemistry days.
Yeah, runaway chemist. Lol.
You have posted your research article. Good idea indeed
Well, its a summary of it. I recently learnt from Phdforum on twitter that blogging your research article gives a wider view to your work. This is one of the places to do it. Thanks. How are you doing?
Thats really cool.I am good thanx for remembering me..really appreciate..
I am not understanding. The only thing I know is that cyanide is quite toxic.
Yeah. You learnt something of course.
Damn... this one got me confused. 🙈 I will read it again once I am done sleeping. The post is too technical. Kudos turpsy.
Thank you @sweetestglo-eu. It was more of research conducted in the laboratory. :p
Thanks.