What makes wine cry? Let's explore from first principle the "Tears of wine" - A case of Marangoni effect
Marangoni may sound like some spell from the “Legend of the seeker” or “merlin” series or maybe not, but anyhow, you sure will wonder at what I’m about to reveal to you. Now, before you think I’m here to teach some magic tricks like an illusionist, Marangoni is an Italian name and the name of the man who researched and explained the Marangoni effect.
Carlo Marangoni is his full name and he was the physicist who studied this effect for his Ph.D. work at the University of Pavia and published his findings in 1865. However, it was James Thomson in 1855 who first observed this effect in a glass of wine albeit he doesn’t know that it is an instance of a general effect that will later be called the Marangoni effect.
I presume you are filled with curiosity at wine crying and the strange term “Marangoni effect”. Well, that’s why I have struck my computer keyboard severally to explain to you how the wine cries and what makes it cry. Without tarrying further, let’s delve in.

Prolusion
Humans (even the lazy ones) are constantly hustling to make ends meet. Everyone strives to get what they desire, but we wouldn’t do any of this if we have what we need. As a result, it is no doubt that there’s a driving force behind our hustling.
Some will say they are driven by passion, while others are in for the money. Party to the former will look at the latter with disapproval and the latter will say to the former, “Let me see how far you can go”.
Nonetheless, the debate of which is right or wrong is not germane in the course. Whichever league you belong to, you surely have one thing in common with your counterpart – A driving force. This might not be apparent to onlookers, but surely as a governor of your life you know what moves you, be it passion, money or maybe a touch of both, but what is most important is that you have a motivation.
Summarily, there is no smoke without fire and there is no effect without some cause. Thus speaking of driving force, every life’s entity has one form or the other making sure such entity exist as it is. Basically, what we observe in every matter on the macroscopic level is a manifestation of what happens on the microscopic level. That is, the effect of this covert microscopic interaction is what we perceive.
Every matter is composed of molecules which are interacting which each other, and exerting forces on one another – the repulsive force which is a short range force and the attractive force which is a long range force, collectively known as intermolecular forces. However, of interest to this course is the interaction of molecules in liquids.
The repulsive force is as a result of electron cloud of the molecules. Recall that molecules form as a result of bonding between atoms and this bonds are formed by virtue of the need to attain stability. One species, the most electronegative takes electron from another species, which is more electropositive. The core of this molecules is thus surrounded by shield of electrons – the electron cloud.
It is well established that electrons carry negative charges and likewise, the principle of like-pole repulsion is renowned. Consequently, bringing two molecules closer results in some repulsive action as a result of their electron clouds. Obviously, the closer the molecules get, the stronger the repulsion and hence, short-range.
What about the attractive forces?
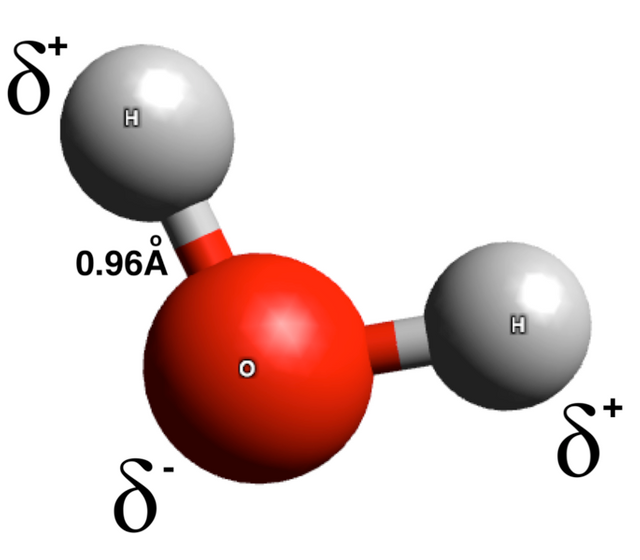
The force of attraction on the other hand is as a result of partial polarity at the ends of each atom present in the molecule - this is denoted by small delta sign. We expect a molecule to be neutral, well that’s not completely true as each atom present within the molecule have different affinity for electron and thus pulls differently on the electrons shared compared to its counterpart.
Water for instance is composed of two hydrogen atoms and one oxygen atom. Like, I said earlier, the aim of each participant in the resulting molecule is to achieve a stable electronic configuration. Oxygen requires two electrons to achieve this and hydrogen requires only one electron to become stable. Hence, it takes two hydrogen atoms to satisfy the need of the oxygen atom and vice versa.
The electron affinity of oxygen exceeds that of hydrogen so that the oxygen atom will pull more on the shared electrons than the hydrogen atoms. It’s like two people, say brothers having equal rights to a thing, say a piece of cake, the eldest will want to take a larger share of the cake all in the name of being older.
The oxygen atom in water pulls stronger on the shared electron and thus acquires a partial negative charge. It’s like taking a larger share of the cake. On the other ends, the hydrogen atoms are inferior and cannot match the strength of the oxygen atom, thus they give away some of their rights, that is, settling for the lean share of the cake. Hence they inherit a partial positive charge.
The interaction of this partial positive and negative charges at the ends of various molecules in the bulk of the water results in attraction between the molecules. This attractive force spans over a long range than the repulsion resulting from the interaction of electron cloud. This long-range attractive force is known as cohesion.
Similar to cohesion is Adhesion. Both are attractive forces, however, the former exists between molecules of the same species, while the latter exist between molecules of the different kinds. Cohesion and Adhesion play important roles in the nature of liquids and water is a perfect case to review.
Have you observed that water clings to the wall of a glass tube? That's adhesion in action. Water molecules have a stronger affinity for glass molecules than they do towards each other. As a result of this, the surface of water when inside a glass tube will not be level. Portions nearer to the walls of the glass will be higher forming a concave shape known as a meniscus. Furthermore, this adhesion is responsible for the rise of water in a narrow glass tube - a phenomenon known as capillarity.
There's more to Cohesion
Let’s go back to the molecular level. Picture some water in a small container, say a tea cup. In the bulk of the water, molecules are interacting with one another. Each molecule experiences an attractive force from every other molecule surrounding it – remember the cohesive force. For the sake of clarity, each molecule can be assumed to contribute equally to the force felt by the molecule in question and also the system is isotropic and thus all these forces cancel out, leaving no net attraction on the molecule in question.
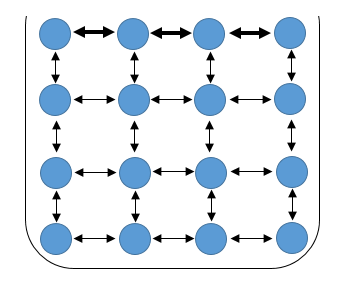.png) Schematic showing the Cohesion in water at the molecular level (image created by me - @temitayo-pelumi)
Schematic showing the Cohesion in water at the molecular level (image created by me - @temitayo-pelumi)However, we cannot say the same for the molecules at the surface of the water, they have molecules of their kind below them, but they are interacting with another species just above the water surface. Consequently, the cohesive force felt by these surface molecules is from those below and beside them. Now, since there are less contributors to the force felt by such molecules, each molecule pull stronger than those interacting with a molecule in the bulk and there’s a net force on such molecule in a downward direction.
To better understand this, imagine if ten people are required to carry an object and a day came where there are only five men available to carry out this same task. It is obvious that each of the five available people will have to improve with their effort, most likely double it.
The molecules at the surface of water (and any liquid) pull each other stronger than molecules in the bulk do. Each molecule experiences a strong pull, which tends to reduce the surface area to the possible minimum. Consequently, interfacial tension is created at the water surface, and this is called surface tension.
It’s like trying to stretch a piece of fabric from every point on its edge although this will result in an increase in surface area but, you can get a better glimpse into surface tension with this. However, the forces resulting in this tension are always trying to contract to liquid surface, thus making the liquid surface denser and at the same time under tension, say an inward stretch instead of that with the fabric.
I believe the concept of surface tension shouldn’t be too strange to anyone of science background but the driving force is probably unusual. Well, that I believe has been dealt with here. But there’s still more, so let’s move on.
Surface Tension as a barrier
Surface tension is a barrier on the surface of a liquid. It’s like when the surface of water freezes in the artic, preventing assess into the pool of water. Although this barrier presented by surface tension offers an infinitesimal resistance to intrusion in to the bulk of the liquid. In a more technical term, surface tension is the force acting on a film of liquid of unit length. It has a unit equivalent to the ratio of force to distance, i.e. N/m.
If you wonder what the physical meaning of this definition is, then worry no more. What this definition is pointing out is that to break the barrier offered by a unit length of smallest possible sheet of a liquid, you require a particular amount of force. Like in the fabric analogy I gave earlier, if you are to tear through a portion of the fabric while it is stretched, you need specific amount of force.
Due to the minute nature of surface tension, it is measured using a lower order unit of force called dyne. One dyne is the force required to accelerate a one gram mass at a rate of one centimeter per seconds. To save you some technical jargon and the need for analysis, 1 dyne is equal to 10 micronewtons. Surface tension is measured in dyne per centimeter (dyn/cm).
With respect to air, that is if air is the fluid above the liquid, water has the highest surface tension after mercury. Additionally, surface tension is a temperature dependent phenomenon. The surface tension of water is about 72 dyn/cm at 25oC, with mercury having a value of 485 dyn/cm at the same temperature.
Surface tension is responsible for some fascinating events observed in water. For instance, a paper clip carefully place on what will float on water, that is, it is supported by surface tension. In a technical sense, the weight of the clip is not enough to break the barrier offered by surface tension. In addition, insects such as water striders are able to walk on water. The spherical shape of water droplets is another effect of surface tension.
The aforementioned events are quite fascinating and wowing but I bet the “tears of wine” will even wow you more. Perhaps you have seen a tear-like pattern on the inner wall of a wine glass containing wine and wondered how this happens or maybe you are totally unaware and what comes to your mind is - why the wine cries, is that even reasonable? Here, we go then.

The Marangoni effect and Tears of wine
Without lingering, the Marangoni effect is a surface tension gradient induced flow and this theory concludes that molecules of a liquid will drift to a region of higher surface tension. The rationale behind this is the fact that regions of higher surface tension pulls better on molecules. It’s like a tug of war where the stronger team wins.
So, if there occurs variation of surface tension in a pool of a liquid, the region with higher surface tension will pull better on the molecules at the surface and the liquid will flow towards such direction.
This said surface tension gradient or variation may be as a result of changes in temperature or concentration of the liquid.
Let's see Marangoni effect in action
As they say, seeing is believing. Hence, to make this explicit and interesting, I have decided to show you a brief science wonder, a little experiment showing the Marangoni effect in existence. This experiment is what you can easily practicalize at home with domestic supplies.
The ingredients for this experiment include some water, solid particles (I am using the dried thyme seasoning powder), surfactant (I am using soap dissolved in water in high concentration, a dish detergent will also do the trick), cotton buds, and a wide dish. Here’s how you carry out the experiment.
Pour some water into the dish, sprinkle the thyme powder into it until you have enough on the water surface. Now, dip one end of the cotton bud into the surfactant and leave the other end pure. Okay, we are ready for the show.
We will begin with a confirmation. Dip the pure end of the cotton bud into the center of the dish. What do you observe? Well, nothing. You will understand why I call it confirmation soon enough.
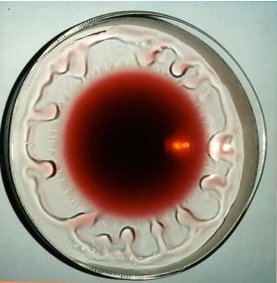
I guess you already know the next thing. Of course, dip the other end of the cotton bud with surfactant into the center of the dish. Now, something happens. The thyme particles drift radially away from the center towards the edge of the dish. So what really happened?
Surfactants are substances that reduce the surface tension of a liquid. Dissolving some soap in water reduces the surface tension of water. Remember that the water here is that which we dissolved some soap making it a soap solution and thus water with lower surface tension compared to a pure water. Do not confuse it with the water in the dish.
Now, here is what happened in the experiment. Dipping the pure end of the cotton bud into the water in the dish does not alter the surface tension of the water since we have only introduced more pure water into the dish. However, using the other end of the cotton bud which holds the soap solution, you will observe that the thyme particles drift away from the center.
This is due to the reduction of the surface tension at the center of the water surface in the dish. Since regions of high surface tension pull better of the liquid molecule, the molecules will be pulled away from the center were the surface tension is lesser and hence drift away. As more soap solution enter the center with time, it further reduces the surface tension and the molecules drift farther away from the center.
The thyme particles are mere indicators here, to show you that the molecules at the surface of the water drift away from the center. In other words, the reduction of surface tension at the center resulted in a flow towards the rim of the dish. However, this wouldn’t have been noticeable without the thyme particles. But the question still remains, how does this make wine cry?
The Tears of wine proper
Basically wine contains water and ethanol (alcohol), each having different magnitude of surface tension, with water having a higher value. Nonetheless, ethanol is a liquid with a higher volatility than water and thus evaporates at a faster rate than water. A mixture of both water and ethanol gives rise to another liquid with a different surface tension and the more the ethanol the lesser the surface tension and vice versa.
That is, the concentration of ethanol in the wine solution decreases due to the evaporation of the ethanol content. If you can recall well, I noted that the Marangoni effect is due to surface tension variation and I also mentioned that this variation could be from changes in concentration of the liquid and such is the case here.
Tears of wine is initiated by a thin layer of wine on the wine glass. This layer of wine is able to stick to the glass due to adhesion, especially when you swirl you wine glass before taking a sip. Evaporation of the wine ethanol content occurs throughout the free surface including that of the thin layer.
Due to this evaporation, the ethanol content of the thin layer of wine is lesser than that in the bulk. Thus, surface tension is greater in the layer than in the bulk. As dictated by the principle of Marangoni effect, fluid flow will occur towards a region of higher surface tension.
Consequently, wine flows from the bulk and climbs until reaching the top of the thin layer of wine, defying the law of gravity and forms a ring around the wine glass. At this point, the wine accumulates into a ridge which thickens with time.
At some point, the fluid accumulates no more and its weight reaches a stage where it is overwhelmed by gravity. At such instance, the accumulated fluid begin to roll down like tear drops from human eyes and hence, the Tears of wine. Additionally, these dripping droplets of wine but is also called other names such as wine legs, curtain and church window. A plan view of a wine glass depicting this phenomenon is shown.
There are claims that wine tears is an indication of wine quality. However, this is a misconception as it is only an indication of surface tension gradient, that is, the Marangoni effect. Nonetheless, the amount of tears is an indication of the ethanol content of the wine – the more the ethanol in the wine, the more obvious the wine tears will be.

Closing
Like the rainbow in the sky, many physical phenomena are a wonder to behold. We are often meted with confusion as to how they came to be and sometimes constantly asking ourselves what drives their existence.
However, science is always seated behind some walls, trying to unravel these things we perceive as mystery. While some have been unraveled and some only hold a potential to be as work is still in progress, others are still inexplicable.
Nonetheless, albeit it is most likely that at hearing “Tears of wine” one could be bewildered, science has added its driving force to its archive of success and I want to believe you have been able to grasp that here today – So, I guess you should scrap that out of your mystery list.
Therefore, next time you are at the gathering of friends and they wonder at this occurrence, I’m sure you will be the geek of the day, wowing them with the science behind it.

Acknowledgement
Special Thanks to John W. M. Bush, Professor & Associate Head, Department of Mathematics, MIT for granting the permission to use an image from his publication.

Reference
- Venerus, D. C. and Nieto Simavilla, D. Tears of wine: new insights on an old phenomenon. Sci. Rep. 5, 16162; doi: 10.1038/srep16162 (2015).
- Podgornik R. and Matja Z. (2014). A basic macro- and microscopic view of surface tension and capillarity. Faculty of Mathematics and Physics, University of Ljubljana.
- KHANACADEMY: Cohesion and Adhesion of Water
- USGS: Surface Tension and Water
- BC-Campus: Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action
- Wikipedia: Tears of Wine
- Surface Tension
- Surface Tension Values
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details.

I'm a proud member of @promo-mentors where you get mentored and guided on how to make quality posts on steemit amongst other benefits. Do join us on discord
We anticipate your arrival.


I usually just swirl wine to pretend that I know what I'm doing. Now I have some inkling of why.
Good to know that the article was able to provide the answer to your curiosity. Thanks for dropping by.
Congratulations @temitayo-pelumi! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPThis is beautiful writing @temitayo-pelumi, especially the begininng.
Thanks dear. We have been given much on this platform and the least we can do is to contribute a good Quota of writing.
Thanks for dropping by.
Now I get to learn something new. The video was magical!
The Marangoni effect is for sure a wowing phenomenon. I'm glad you found it fascinating.
Thanks for reading.
Really well explained article, bro. Didnt know much about Marangoni effect before reading this. That video was cool too!
Cheers!
It is fulfilling to know that you were imparted by this article. I believe coming from a rudimentary perspective would makes things clearer for the readers and its good to know that it did for you.
Thanks for reading through.
u r most welcome :)
Hi @temitayo-pelumi!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @temitayo-pelumi! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPNice post, pls keep it up.
pls follow and upvote me on my below post too
https://steemit.com/photography/@harrysaini/scribbling-art-series-2
https://steemit.com/painting/@harrysaini/scribbling-art-series
Thanks.
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Nice one, i have learnt something great today.