Basic Knowledge Of Ozone layer (stratospheric layer)
 Ozone layer
Ozone layerKnowing very well the different layers of the atmosphere will help us to have more knowledge of the ozone layer. There are different layers of atmosphere which are troposphere and stratosphere. In the troposphere (first layer), small aircraft flights, mount climbing and gas balloon are the activities that take place there. This layer is 10km away from the earth surface.
The second layer is stratosphere and at the lower end of this second layer is ozone layer that is 20km above the surface of the earth. The ozone layer thickness varies according to geographical region and fluctuate based on the season and its thickness is always between 3mm-5mm.
Ozone layer
This layer is found in the Earth upper atmosphere of stratosphere and it is the layer in which the ozone is embedded in. Ozone is gaseous in nature as it is made up of three oxygen atoms (O3).The part of stratosphere in which ozone is embedded is referred to as ozonosphere. 0.3 parts per million is the average concentration of ozone in the atmosphere while the concentration of ozone in the ozone layer is about 10 parts per million. At altitude from 26 to 28km, the highest concentration of ozone could be found in the tropics and at 12 to 20km towards the pole.
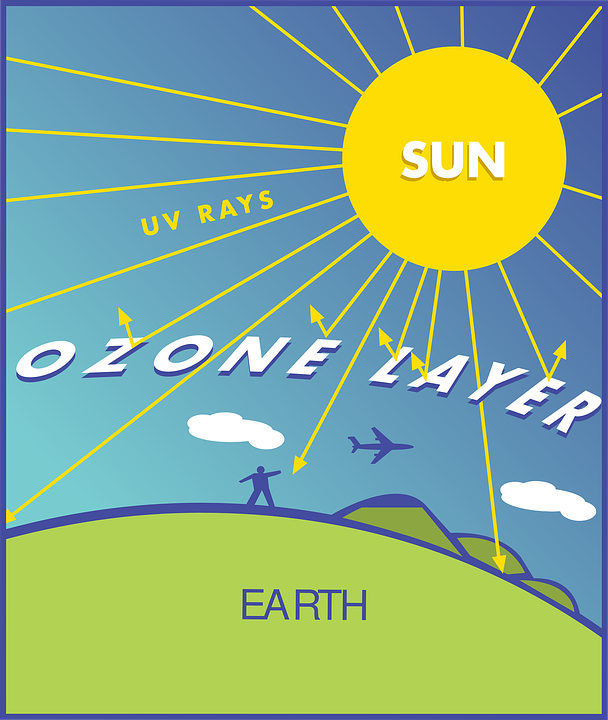
Ozone layer illustration
French physicists that discovered ozone layer in 1913 are Henri Buisson and Charles Fabry. Ultraviolet rays are very strong radiations that come from the sun and are very harmful and life threatening. These ultraviolet rays are being covered by the ozone layer from reaching the earth.
The effect of exposure of human to these ultraviolet rays include; immune system damage, skin cancer and cataracts. Ultraviolet rays possess power to destroy aquatic ecosystem, terrestrial plant life and single cell organisms. These long term effects of ultraviolet rays on human beings, plant and animal is being averted by the ozone layer because this layer possesses the ability to absorb up to 97-99% of the harmful radiations.
Causes of ozone layer depletion
Many of the causes of ozone layer depletion have been attributed to human activities, especially human-produced chemicals. These chemical are known as Ozone-depleting substance (ODS). The chemical termed halocarbons which contain chlorine, fluorine, carbon and hydrogen are ozone depletion compounds.
Human-produced depletion gases include cholofluorocarbon (CFCs), methyl chloroform, hydrochlorofluorocarbon (HCFCs) and carbon tetrachloride. These gases are eco-friendly and their application could be found in areas like air conditioning, refrigeration, foam blowing, as solvent and cleaning of electronic component.
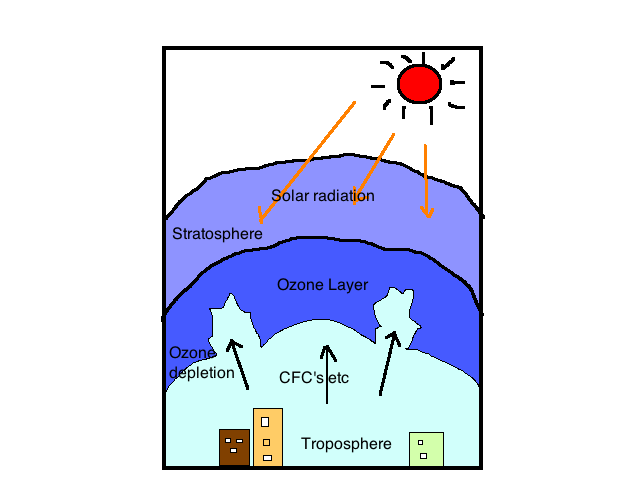
Ozone layer depletion
Apart from human –produced chemical that results in ozone layer depletion, we have natural depletion of ozone layer and this happens as a result of natural phenomena such as stratospheric wind and Sun-spots. This natural depletion causes not more than 1-2% depletion and it is believed to be temporary.
Effect of Ozone layer depletion
Damage to human health
Ozone layer depletion results to over exposure of UV light which results into skin cancer, cataracts, quick ageing and immune system weakening
Treat to marine life
Over exposure of UV radiations to the aquatic lives threaten their survival. For example planktons which appear up the aquatic food chain is affected by exposure to high UV rays which results into decrease in the number of planktons, thereby disrupt the marine food chain.
Environmental Devastation
Many crops species like wheat, corn, oats, barley, broccoli, tomatoes, rice etc. are vulnerable to overexposure of strong UV light which results into minimal photosynthesis, growth and flowering.
Impacts certain material
Over exposure of strong UV light to materials such as plastics, wood, rubber, fabric etc massively degrade them
Solutions to Ozone depletion are mentioned as follow
Desist from using pesticides
Limit the numbers of private vehicles on the road
Desist from use of harmful nitrous oxide
make use of product that are environmental friendly
If only we can desist from using these items..
@tpicric1, you are very correct
Ohh that really good to know thanks for sharing
You are welcome. Please kindly upvote the post