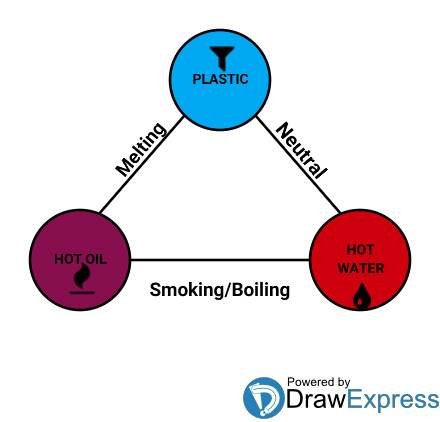
A triangular approach to the reason plastic melt in hot oil but not in boiling water
Few days ago, I had an interesting experience which I think I should share with you guys. It was the saga of a plastic spoon and an ignited frying pan with fried Plantain and hot oil in it. Actually, I was frying some plantains in a frying pan of palm oil in the kitchen and by the time it was done, I couldn't get a clean steel but plastic spoon around me to empty the pan of the fried Plantain into a plate.

But, I thought if I should use a plastic spoon while the pan is still on fire, the plastic spoon would definitely melt because that have been a common occurrence on my side. As a result of this, I decided to put the pan containing the palm oil and the fried Plantain on the ground before using the plastic spoon to remove the fried plantain from it with the intention of avoiding the spoon from melting in the act.
Unfortunately, immediately I dipped the spoon inside, the plastic spoon still melted but this time I was a little bit surprised and at the same time curious to about why the spoon melted despite the fact that the frying pan is no more on fire.
Then I came to a conclusion that the common occurrences of the plastic spoon melting as such under similar conditions as stated above actually happened because it was in contact with the hot oil and not because of the heat from the fire makes it like that or because the plastic spoon physically touching the hot frying pan on fire.
If we are very good observers, then we would have noticed that it is the other way round when we are talking about the situation of any forms of plastics in hot water which we might have experienced in various ways domestically.
But, have you ever thought about it that; why did that plastic spoon melt in the hot palm oil and not in hot or even boiled water ? Why is it that a plastic doesn't melt in hot water but melt in hot oil? Well, I urge you to please take off anything bothering your mind right now and put your total concentration in this, as we will be discussing the real reasons behind this honest observation within the next few minutes.
[Image source - Creative Commons; Attribution-Share Alike 3.0 Unported]
I am sure most if not all of us have seen ice change to water. This is exactly what I mean by melting and the temperature at which the ice changes back to water at one air pressure is referred to as the melting point of the ice. Same thing applies to plastic accordingly.
Polymers of different types have different temperature at which they melt at air pressure. When you heat most plastics, it becomes soft and then can be molded or shaped into different forms. The melting point of some plastics are listed below:
Low density polyethylene - 110°C
High density polyethylene - 130°C
Polypropylene - 176°C
Polyvinyl Chloride (PVC) - 75 to 105°C
Polyvinylalcohol - 240°C
Polystyrene - 100°C
Acrylonitrile butadiene styrene - 125°C
The water we are talking about in this case is a clear liquid under room temperature and pressure. At air pressure of one or at sea level, water has been known to boil at 100°C temperature. The boiling point of water is the temperature where liquid changes to gas.

The kind of oil we are talking about in this situation are not petroleum-based liquid used as fuel or lubricant, but the ones that are gotten from plant, animal, or synthetic fat for cooking in our various homes, such as palm oil, vegetable oil, Corn oil, Soybean oil and the likes.
Since the boiling temperature of common oil is something that seems unachievable domestically, then I think we should just limit this discussion to the smoking temperature of oils rather than its boiling point which is far greater than their smoking point. Smoking point of oil is the temperature at which they give out smoke under a defined condition. The smoking point of some common oils are listed below.
Refined coconut oil - 204°C
Palm oil - 235°C
Refined olive oil - 199 to 243°C
Soybean oil - 234°C
Vegetable oil blend - 220°C
The smoking point of Palm oil is shown above to be 235°C, consequently, its boiling point has been estimated based on research to be about 300°C. The smoking temperature of other kinds of oil can be seen here.
I will try connect and bring into limelight the hidden triangular relationship between the melting point of plastics, boiling point of water and the smoking point of oil, plis I would try as much as possible to keep this simple and sweet.
The presence of chemical bonds determines the temperature at which different liquid boils. The chemical bond binding water is not as strong as that of oil. This is so because the smaller molecules of water lack enough surface to get attached to one another. As a result of this, a lot of heat is required to breakdown the chemical bonds present in oil, thereby, making oil to possess a higher boiling temperature than water.
It has been earlier proved that oil has a very high temperature than water which can not be hotter than its boiling temperature of 100°C under normal condition.
Thus, I think it's quite clear enough that the main reason behind the issue of plastic melting in oil and not melting in water is simply because; the temperature at which most types of plastic melts is a lot higher than the temperature at which water boils and averagely lower than the temperature at which the different kinds of oil smoke or even boils.
The plastic spoon earlier talked about in the saga of the frying pan, the hot palm oil and the fried Plantain is a spoon that is probably made out of a type of plastic known as polypropylene which has a melting point of 176°C. The hot palm oil probably had a smoking temperature of 235°C or something close to that.
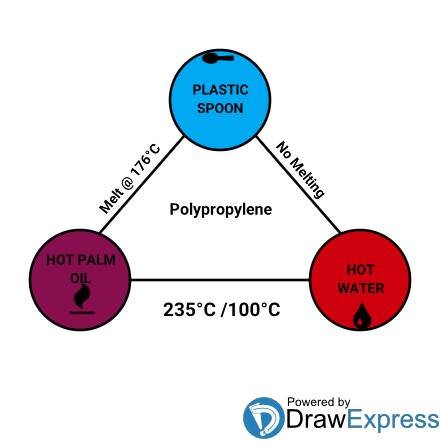
However, some plastic have a temperature that is lower than the boiling point of water such as polyactic acid. As such, this type of plastic can at least become flexible enough in boiled pure water to be able to mould it into different shapes.
- What is the boiling point of water ?
- smoke point
- Plastic
- melting point
- How spork is made
- Boiling oil and water
- At what temperature does plastic melt (Celsius)?
- How long would it take for plastic to melt if you put it in boiling water ?
- Classes of polymeric materials by Professor Joe Greene; CSU, CHICO
It's quite interesting to be a bonafide member of the steemstem community. If you write STEM (Science, Technology, Engineering and Mathematics) related topics, kindly join on on our discord channel here
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
That was quite an interesting observation. Thank you for sharing.
Thank you very much sir
I appreciate the explanation better with the use of boiling and melting point. Job well done @olasamuel
Thank you very much
Congratulations @olasamuel! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPThis is an interesting piece. I actually guessed the answer but just read through to be sure.
Yeah its just as simple and direct as that.Thanks for stopping by