The Ozone Layer: The Thing Between You and Harmful Ultraviolet Light
Space is very near us, an aeroplane flying 11km above sea level already have 75% of the air in the atmosphere underneath it.
At an altitude of 20km above sea level, the pressure of the atmosphere decreases such that water could conveniently boil at body temperature.
At that altitude, the human body experiences the same effect as someone in space. The air pressure is low due to fewer air molecules per given similar surface area at lower altitudes. The higher one climbs, at about 400km, the atmosphere reduces. Here charged particles dislodged from sun's surface creates the aurora (ionosphere).
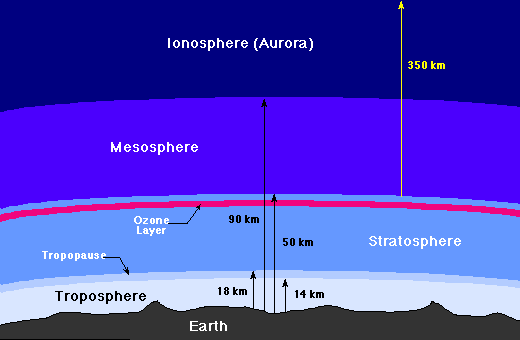
The atmosphere at this height is just a flimsy cover.
How did we get ozone layer?
The geologist believed that ozone layer, as we currently have it, started slowly forming some 2500 million years ago. Before that, the solar UV ray shower made earth impossible for any life form.
Ozone density
If you fly 100km into the atmosphere, you would be at the edge of space. If you climb higher than this, you have officially become an astronaut.
Another interesting fact to know is that almost all the air in the atmosphere ( 99.99997%) lies below this 100km level a.k.a edge of space.
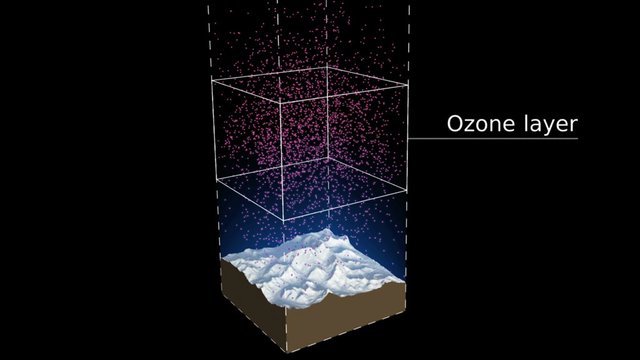
Take a look at the distribution density of ozone. You can see the portion of the atmosphere marked with more dots signifying massive ozone presence and marked by the square box. This higher area of density is at about an altitude of 26km in the stratosphere.
If all the ozone could be extracted as an individual entity from the surrounding other molecules, atoms, etc. and summed together and brought to the sea level. The ozone layer would have been 3mm thick. That just goes to show how delicate the ozone layer is when viewed as a single entity.
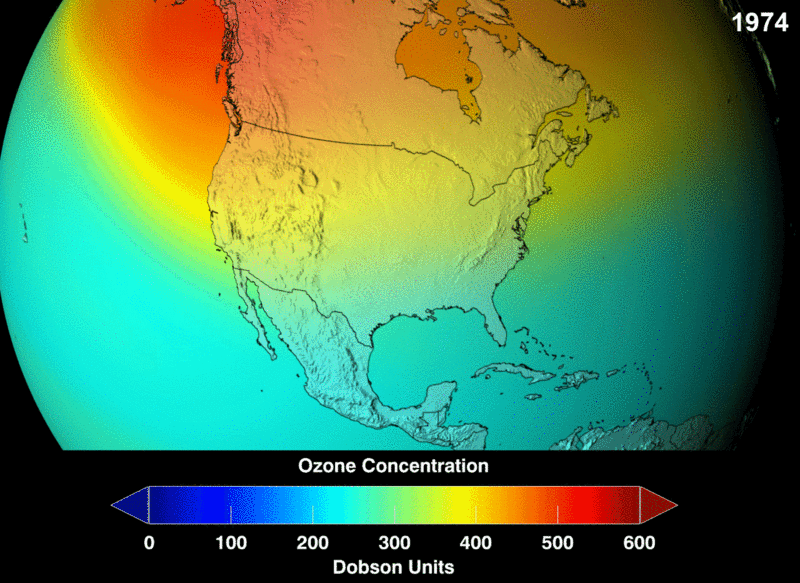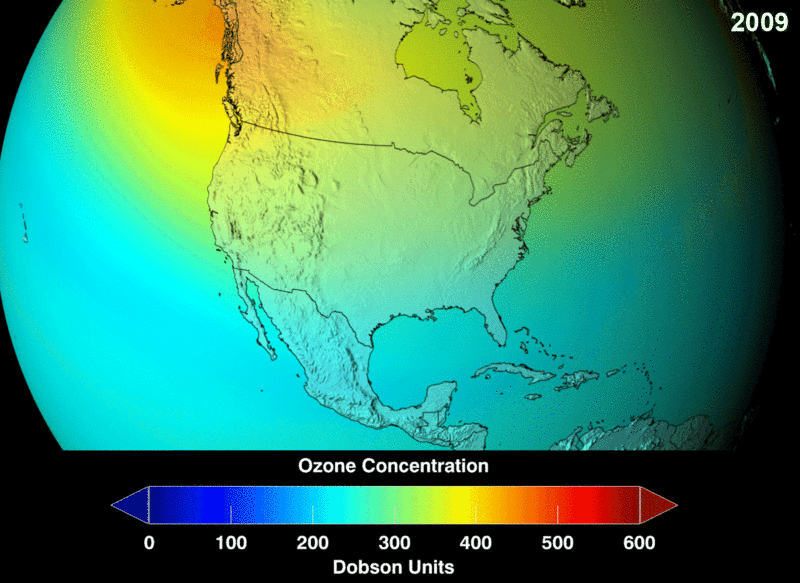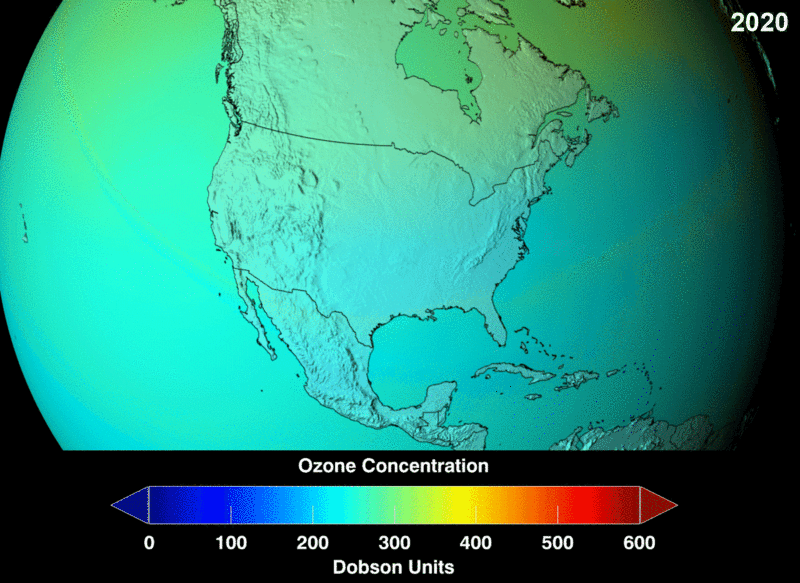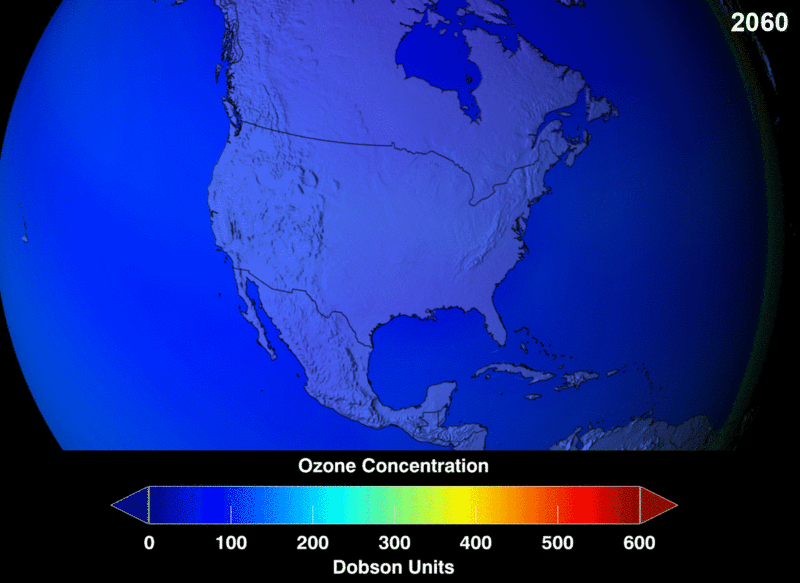
The measure of ozone abundance is done in Dobson Unit (DU). The whole earth has about 300 Du which corresponds to a thickness of 3mm. Still, not much ozone left. From NASA prediction, if CFC use was not banned, this is a visual image of the prediction of how the ozone layer will get to become.
Ozone Layer Cycle
Process 1: The Creation
Ultraviolet light splits an oxygen molecule to form two oxygen atoms. The process of separation of these molecules by light is known as photolysis.
O2 + UV → 2O
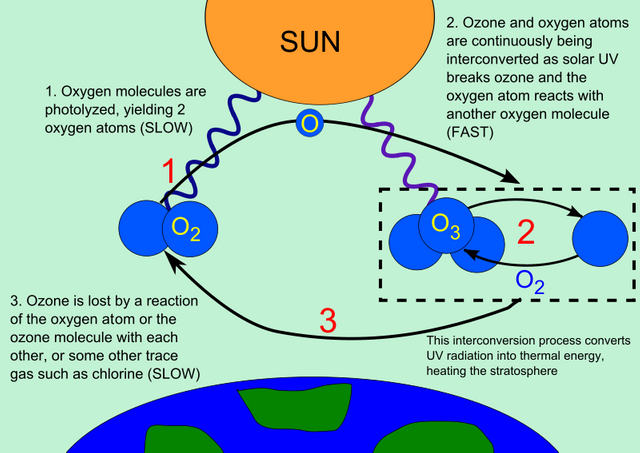
Wikipedia
Process 2: The two atoms formed reacts with an oxygen molecule to create a new ozone molecule O3
O+O2 → O3
When the newly formed ozone molecule reacts with the UV light of between 240nm to 310nm wavelength:
O3 + (240nm < UV-B radiation < 310nm) → O2 + O
The single oxygen atom (O) combines with another oxygen molecule (O2) to form another ozone.
O+O2 → O3 + K.E
Where K.E= kinetic energy. The K.E is the surplus energy of molecular motion resulting from the reaction. It is this kinetic energy that is released as heat in the stratosphere which forms the primary heat source in the stratosphere area.
That continues back and forth reaction yields either ozone or oxygen molecules and is referred to as the ozone-oxygen cycle.
The harmful short wavelength ultraviolet light (UVB) is thereby converted to heat, keeping the ozone layer intact without any overall loss.
Process 3: This is the removal process of ozone. When oxygen atoms (O) combines they form oxygen molecules O2.
2O → O2
This process of splitting oxygen molecules to form 2 oxygen atoms which recombine to form ozone is made possible by the solar radiation in the stratosphere and its removal. The amount of oxygen atoms (O) is usually low since its removal rate is not fast (slow) hence a balance of the ozone layer is maintained.
But the presence of a catalyst such as chlorine (Cl), hydroxyl (OH), bromine (Br), nitric oxide, speeds up this recombination reaction which depletes the ozone layer.
These catalysts are not naturally occurring radicals. They are as a result of chlorofluorocarbons (CFCs) emissions. The free radicals of chlorine and bromine can speed up thousands of ozone removal reactions before it is eliminated from the atmosphere.
A brief history of CFC
The era of the advent of refrigeration of the late 19th century and early 20th century has toxic gases as agents in the process of refrigeration. These toxic gases include methyl-chloride gas (CH3Cl), ammonia (NH3), and Sulphur (SO2).
These are toxic gases, but a game-changing non-toxic gas was soon to be invented. By 1928, a General Motor inventor, Dr Thomas Midgley Jr, an industrial chemist developed a non-flammable and very much sort non-toxic gas known as chlorofluorocarbons.
Its patent name was freon, and it makes up the composites found in fridges refrigerant gases, insecticides aerosol, airconditioners refrigerant, spray paints, etc.
During the peak consumption of CFC, there was about 1 million metric tonnes of it made annually.

The CFCs are non-toxic at sea levels. In the stratosphere, photolysis by UV light splits the CFC releasing chlorine (one of the free radicals). Chlorine is a threat to the ozone molecules (O3) as it yanks an oxygen atom out of it and forms chlorine monoxide with oxygen left behind destroying the ozone molecule in the process.
The chlorine monoxide experiences photolysis too and reaches for another oxygen atom of ozone for stability thereby destroying another ozone molecule. This process continues to repeat itself for some time with each chlorine radical engaging in decomposition reaction with ozone till the chlorine goes out of the atmosphere.
Ozone layer depletion
The ozone layer was apparently fine in between studies of it made between 1977 to 1980. But there were noticeable changes of something not right in 1981. Not until 1984 was a big hole noticed in the ozone layer. The cause of it was traced to CFC use, and on Sept 16, 1987, the Montreal Protocol started a movement to ban the use of CFC.

Logos such as these started appearing in containers of insecticide aerosols, refrigerants, etc.
The thing is CFC is a sneaky pollutant. It could hide in the atmosphere between 40 to 150 years after getting there. The chlorine there could either react with methane and form hydrochloric acid (HCL). This form can rain down to earth. But it is not all bad news.
The Good News
Ozone can self-heal. Oh yes! It is only able to do this because of the ban on the use of CFC in products initiated by the Montreal Protocol meeting outcome in 1987.
Though volcanic eruption does add to ozone layer depletion, the major cause is human-made. Hopefully in the next two decades (2040), we may have an entirely restored ozone layer.
Should we care about this ozone layer?
Yes! We should. It is the earth's harmful UV protector, a sort of UV sunshade. We may be susceptible to diseases such as skin cancers, failure of the immune system, cataracts, etc. if this shield continues to deplete.
The air quality we breathe is also dependent on how well the ozone layer does its job.
References
NASA: Ozone Layer Earth Observatory
Image References
Image 2: Ozone Density Distribution
Image 3: NASA Future Prediction of Ozone depletion if CFC was not banned:
Image 5: Gif showing chlorine attacking ozone
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem.

its interesting this small part of the stratosphere keeps us from harmful cosmic radiation and we are not even concern that our green house gasses are slowly eating and destroying it, we need to look for more ways to protect it or create new way was of replenishing it
You know, it never occurred to me that the ozone layer was never always there; that it only developed 2500m years ago. This very fact emphasizes our need to protect it. The ozone layer is our lifeline, and we seem to be letting it slip away.
If we could only stop harming it, that would be very welcomed development as nature knows how to take care of itself.
Ozone can self-heal. Oh yes! It is only able to do this because of the ban on the use of CFC in products initiated by the Montreal Protocol meeting outcome in 1987.
This gave me some sort of hope because we haven't really been working towards ensuring that we don't emit most of these gasses into the atmosphere, especially in Nigeria and Africa generally. Thanks for sharing this information.
Ozone layer is needed anywhere in the world where the sun shines. Thanks for reading.
The ozone layer has been exposed with time,
Greater damage will be unbearable for living things.
Nice one you put up here..
Looking up for next posts
Thanks for dropping by.
Beautiful chemistry there. We need to educate more people to preserve the earth the least we can do. Plant a tree or a flower in your surrounding. Thank you for the post.
Thank you too.
This is a very interesting topic especially for those who are not in the scientific terrain.
Ozone layer depletion is something that should be of concern to everyone but unfortunately, three decades after the Montreal convention a lot of products with Cfc still flood the market. I worry where we are headed.
Most of the substance that are not ozone or eco friendly were banned. Though some still make use of it where regulations are lax. Thanks.
Are there other man-made reasons besides CFC that cause harm to the ozone layer. Or is the current rate of depletion still related to some CFC release?
I really enjoyed your post @greenrun, good stuff.
Yeah, but the CFCs are the major culprit. Others may include Methyl ChloroformCarbon, Carbon TetrachlorideHalons and halons.
What a wonderful piece of information is this ! Concerning ozone layer.As i go through your work ,i learnt how ozone form ,how it can be destroyed by free radical of chlorine release by CFCs decomposition ,the negative effect of ozone depletion such as skin cancer, loss of immune system and loss of good quality of air and little knowledge about acid rain through combination of methane and chlorine radical.Truly, i am happy to come across your post ,very informative and educative.may God give you more inspiration to affect our life more in jesus name.
Thanks for reading.
Seriously without the ozone layer, it will be so difficult for breathe to be realized. It constitute the air which is been circulated in the earth... Thanks for sharing @greenrun baba.
Thank you.
@greenrun ozone layers!
I received teachings of this post in my elementary classes, basic 8.
My teacher only touched the surface of the topic. 😃
I understand deeply now.
This is educative.
I know understand why the sun is scorching hot these days, the air pollution from motor tires here has badly depleted the ozone layers
That is very true; the environment is under heavy pollution by man through industrialisation and urbanisation. But sadly we seemed to have forgotten that nature does not need us; but we do need nature.
hello @greenrun
What a wonderful piece of information you got sir...very educative. Ozone layer is needed anywhere in the world where the sun shines brightly...more knowledge to ur instincts sir...Looking forward to reading ur next posts
#cheers
@vickyrich
Thanks @vickyrich