The Mpemba Effect: A Scientific Paradox on Why Hot Water Freezes Faster Than a Cold One
If you hear someone ask if hot water can freeze faster than a cool one I bet you might likely go with the cool one freezing more quickly. But if you do that, you may be in for a big surprise as what happened in 1963 proved.
In 1963, a 13-year old Magamba Secondary School pupil from Tanzania, Erasto B. Mpemba, accidentally discovered that the weird behaviour of hot liquid and its freezing behaviour compared to cool liquid. That discovery rekindled the centuries-old phenomenon that had many scientists perplexed.

Pixabay Image CCO Creative Commons
A little history
In 1963, after a cookery class in ice cream making, Mpemba was in a hurry to put his newly made ice cream in the freezer before other pupils did, and take up all the available space. In his rush, he did not allow his hot milk to cool down before placing his cup of ice cream in the freezer.
When it was time to check on his icecream Mpemba was surprised to see his ice cream had frozen well before others who placed cool ice cream.
When Mpemba told his classmates and teachers this, they laughed it off and said he must have made a mistake, but Mpemba was to have the last laugh.
In Mpemba's words,
When Dr Osborne visited our school we were allowed to ask him some questions, mainly in physics. I asked: “If you take two similar containers with equal volumes of water, one at 35 °C and the other at 100°C, and put them into a refrigerator, the one that started at 100 °C freezes first. Why ?“ He first smiled and asked me to repeat the question. After I repeated it he said: “Is it true, have you done it?” I said: “Yes.”
Then he said: “I do not know, but I promise to try this experiment when I am back in Dar es Salaam.” Cool
The visiting physicist, Prof Denis Osborne (PhD) was intrigued by Mpemba's observation and had it tested in the lab. Well, let us just say he validated the claim and Mpemba had the last laugh.
The duo published a paper in 1969 called Cool A paper by Erasto Mpemba and Denis Osborne which you could read some excerpts of it here.
Mpemba was not the first person to have noticed this phenomenon, Aristotle, Francis Bacon, and René Descartes had similar experiences.
Aristotle first discovered this phenomenon in 4th Century AD that heated water freezes faster than water that is cold. Later, other scientists like Francis Bacon and René Descartes observed the same thing.
Many people have come up with their explanation why hot water should not freeze faster than hot water. They all have a proof for this phenomenon. One of them is that say you have water at both 30°C and 70°C respectively. You start cooling both at the same time. The 30°C water may take ten minutes to freeze while its warmer neighbour starts off at 70°C. The 70°C water will take a longer time to cool down to 30°C and then take another ten minutes (the same time it took the 30°C water) to freeze. I can hear many saying "Yes, that is correct!"
Well, Mpemba will like to tell all of us that assumes above to be correct to wait a moment or like my elementary English teacher would say, "Hold your horses!"
The proof above only considers one property of water in this assumption: its temperature.
It negates the fact that there may be other factors, besides the temperature. What if the 70°C water experiences other changes as it is cooling to the 30°C? There is a probability or tendency that the mass is affected (less mass), convection current creating an uneven temperature distribution or even the water having decreased dissolved gas.
One Effect Different Results
A seemingly simple phenomenon had taken a long time to be explained due to the nature of water. There are many details which affect how a water freezes and these may change a lot of experimental setups. These include impurities and gas contents in the water, size and shape of water in both container and refrigeration unit, the mapping of time of freezing, etc.
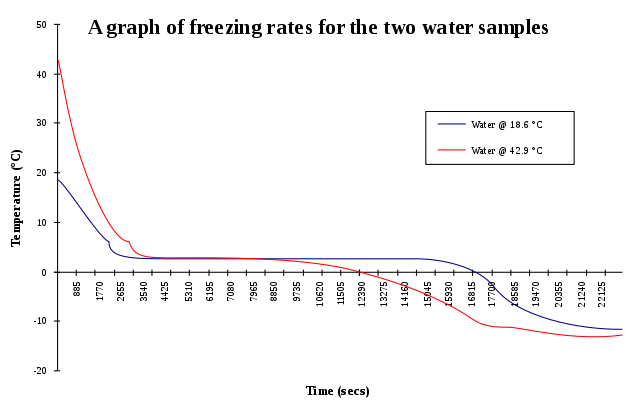
[Wikipedia Creative Commons Attribution 3.0. Author: SVG]Source: Graph of freezing rates for two water samples to demonstrate the Mpemba effect
While many scientist's experiments have shown the existence of the Mpemba Effect, there has been this disagreement over the conditions which makes it occur.
Theories for Mpemba Effect
No one mechanism explains Mpemba phenomenon for all circumstances. Therefore, listed below are some of the mechanisms that explain the conditions for Mpemba Effect to occur.
Evaporation: There is considerable loss of water due to evaporation as the warm water loses temperature to get to a lower temperature ( from 70°C to 30°C in the above hypothesis). This rapid loss of water makes for a reduced mass and hence quicker freezing albeit with less ice compared to the cooler water.
This explanation is excellent when evaporation is the only criteria to explain the Mpemba Effect. But it will hold no water (pardon the pun) if the experiment is done in a closed container where there is zero loss of mass due to evaporation.Dissolved Gases: There is less amount of dissolved gases in hot water as it escapes during boiling. This property of warmer water is thought to make it easier for the convection current to develop leading to the faster cooling of warm water. There is a decrease in the amount of heat needed to freeze a unit mass of water.
Convection Current: There is a phenomenon known as "hot top". It is as a result of the uneven temperature of water due to convection current as the water cools. The relationship between density and temperature is such that temperature increase causes a reduction in density of water. This produces the phenomenon of hot top where the top of the water is warmer than its bottom. The warm water cools quicker than the temperature of the cold water, but due to its hot top, it rapidly loses more heat than the initially cool water.
There have been many instances of hot water freezing before the cold one, but there is universally binding agreement or cause or circumstance that led to such an effect.
Now let is get back to the present, in 2012, the Royal Society of Chemistry decided to put this phenomenon to scientists. They placed a cash price of £1000 to any person or group that could come up with the best creative explanation of Mpemba Effect.
There were 22,000 entries from scientists all over the world. Finally, the list was whittled down to 11. On 10th January 2013, a winner was announced.
And the winner is..
Nikola Bregovic, a researcher from Croatia in the Department of Chemistry, University of Zagreb.
What did he do that the other 21,000 plus did not do?
He thinks that convection current is a driving force that explains the Mpemba Effect. The convection current in warmer water makes for a uniform heat distribution.
Well, that is it, folks, problem solved.
NO!
The phenomenon is far from over as explained by some scientists from Singapore who posted an article in October 2013 where they claimed to have "solved" the problem.
They said the problem's solution is in the bond of water molecules. A molecule of water comprises two small hydrogen atoms and a big oxygen atom. But with more molecules together the effect of the hydrogen bond is felt more. Due to the hydrogen bond of water, it has a higher boiling temperature when compared to other liquid with similar molecular structure.
Xi, one of the Singapore scientists, proposed that the hydrogen bond of water, which makes it easy for plants to draw water through its capillaries as evaporation takes place on its leaves, is what pulls the water molecules into proximity. The stretching of the molecules creates a covalent O-H bond which stores energy. Note here; the water is less dense as the liquid warms up, the molecules are spaced wider apart.
This stretched hydrogen bond leads to a more shrunk and relaxed covalent bonds, and this makes them release some energy. When the covalent bonds give off energy, it is the same as cooling. Theoretically, the warm water gets cool faster than the cold one.
There is some calculation showing the significant number of covalent bond relaxation that brings about the rapid cooling time differences between the cold and hot water.
That was a pretty convincing explanation, but honestly, that will not do it to explain the scientific paradox of why hot water sometimes freezes faster than a cold one.
Like Mpemba, we should never stop asking and learning. Who knows if you would be the next to discover an anomalous physics, which can catapult you into the physics hall of fame.
References
- The original story behind the 'Mpemba Effect', as told by Erasto Mpemba and Denis Osborne
- Why Hot Freezes Before Cold One
- Royal Society of Chemistry Prize Won
- Mpemba Mystery Solved?
- O:H-OBond Anomalous Relaxation Resolving Mpemba Paradox
- Unusual Properties of Water
- Singapore Scientist's Explanation of Mpemba Effect
- Why Hot Water Freezes Faster Than Cold-Physicists Solve the Mpemba Effect
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem.

I wouldn't have believed it that hot water freezes faster than cold water. No wonder science discovery. You did some good research here, @greenrun. Well done!
@maryfavour & @redfishpillar.
Thank you for stopping by, and yes science always tries to provide answers to our questions.
Really interesting.. I've heard something close to this but I' m learning about it for the first time...thanks for sharing
I agree it was an interesting conversation. Thank you for reading.
There are indeed crazy science facts we can discover every day.
I have no idea that hot water freezes faster than cold water, it seems to be against logic.
I wonder why would Mpemba even think about experimenting with this? I suppose such is the mind of a curious person
True that
Great content.
You know water display a lot of anomalous behaviour, like not expanding upon heating until it gets to 4°c and so likewise your topic for today, on hot water frezzing faster than cool water.
Thanks for sharing @greenrun
She knows
This is amazing. I never would have imagined it, and I'm glad that the professor published the paper with Erasto. Some professors like my project supervisor would have published the paper without giving credit to the kid.
That is something that shouldn't be done in education; taking someone's idea as solely yours. There should be that team work spirit as no one has the exclusive preserve to out-of-the-box thinking.
Hey @greenrun,
Dude you are good. Mpemba Effect, I'm just hearing that for the first time. I love the way you told the story. Science is a very interesting subject indeed.
A teacher actually told us this in a physics class in secondary school, it made for quite an interesting conversation after the class :)
You guys probably started thinking of what to discover right? Thank God you finally discovered steemit.
Steemit actually discovered me. Lol
Make that the two of us. But hey, maybe you should share that story with us someday.
@pangoli, I always share my stories :)
A perfect example of how even simple questions can lead to amazingly complex answers!
That is very true. Had he not asked or upheld the first answer as the truth, we may probably not get to hear some of these excellent answers.
good idea D :@winstonalden
Shouldn't the answer be obvious though? Hot water has the molecules further apart therefore when exposed to low temperatures, more surface area of individual molecules is exposed to those low temperatures than that of cold water whose molecules are packed much closer together.
Why did you not submit this entry in 2012 and take a shot at the £1000 challenge from the Royal Society of Chemistry? :)
Not too late @edumurphy, you can still submit it :D
They will expect more experimental rigour than I am willing to bother with. Do they take offhanded Steemit comments? That's more my speed 👺
Besides, your post actually shocked me because I always assumed this question had been looooooong answered
It not yet answered. So, here's your chance to give it a shot.
WOW this is one of the best steemstem articles I have read. I can't believe that warm water freezes faster than cold water. And more surprising is that it isn't explained yet..
Great article man!
Hello @futurethinker, it is a pleasure to have you here, thanks so much for the compliment. I have this fascination with the Mpemba Effect, a seemingly simple phenomenon with different explanation.