Water hardness. Do you know what type of water you use or consume?
Maybe, in some occasions you have noticed when washing your clothes at home, the consumption of soap is greater to achieve the same degree of cleanliness, or you have seen the formation of crusts in the bathtubs or incrustations in the pipes.
You would have noticed as well that bottles of mineral water specify in their label the concentration of calcium and magnesium.
Do you know why this happens?
In this article I want to present the answer to that question, so I invite you to review it!
The total hardness of the waters is a very important parameter; its determination provides a measure of the water quality for domestic or industrial use.
Some time ago it was defined as the capacity of cations present in water to displace sodium or potassium ions from soaps in order to form insoluble products. As water moves through soil and rocks, it dissolves minerals and keeps them in solution. However, in natural waters the concentration of calcium and magnesium ions are higher than any metallic ion, which is why Hardness is now defined as the concentration of calcium carbonate which is equivalent to the total concentration of all multivalent cations in the Sample[1]. The degree of hardness is greater as the concentration of these ions increases.
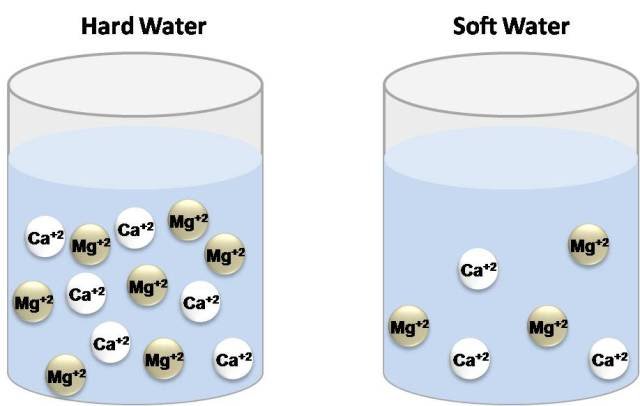
Water types according to hardness. Own image.
Based on this, your observations can be explained
In the case of detergents, the use of hard water decreases its efficiency when insoluble salts are produced, making it difficult to foam and this leads to greater consumption to obtain the same results in cleaning, raising costs and in some cases implies the use of aggressive cleaning agents. Also at the domestic level you can see less shine on the bathroom and kitchen surfaces.

Effect of hard water on foam formation. Owm image.
In the pipes the incrustations take place when the hard water begins to circulate since the lime begins to accumulate. The calcium contained in the waters crystallizes; they adhere to the surfaces producing immediately calcareous solid incrustations that have destructive consequences.
These calcareous incrustations are formed especially in places where high temperatures prevail, such as water heaters, radiators and others. The higher surface temperature, the more lime incrustations will develop, this is because the calcium and magnesium salts are of inverted solubility, that is, their solubility increases with temperature but, upon reaching 60 °C or more, its solubility drops abruptly causing the formation of scale and a loss in the efficiency of the heat transfer, which reduces the diameter and can cause the pipes to clog[2].
Being the calcium and magnesium the main compounds of the incrustations we would expect the calcareous deposits to be white. However, most of these deposits are red. That explains why there is a relationship between calcareous encrustations and corrosion. When the calcareous deposits are formed, the iron and oxidized iron particles are fixed in them, this produces the reddish color. This means, in addition, that the oxide adheres strongly to the ducts causing a serious threat due to corrosion.

Physico-chemical characteristics of water for human comsuption. Own image.
The hardness content in water for human consumption has been extensively researched and evaluated for many years in different studies. However, the World Health Organization indicates that hard water does not have a harmful effect on people’s health. The hardness of the water can influence the taste or the organoleptic properties. In drinking water, the hardness usually ranges between 10 - 500 ppm of calcium carbonate. For WHO, the usual tolerance threshold ranges between 100 - 300 milligrams, although it points out that many consumers accept higher numbers without problems (up to 500 mg / liter)[2]. For this reason, its calcium and magnesium content must be specified in the containers, complying with the quality parameters.
There are two types of hardness, the temporary hardness that is determined by the content of carbonates and bicarbonates of calcium and magnesium. It can be removed by water ebullition and subsequent elimination of precipitates formed by filtration; and the permanent hardness, which is determined by all calcium and magnesium salts except carbonates and bicarbonates. It cannot be eliminated by ebullition of water.
Now, from the analytical point of view, how is water hardness determined?
Total hardness (calcium hardness and magnesium hardness) can be determined by means of a direct evaluation based on a complexation reaction. The formation of a complex involves the reaction between a metal ion M with a ligand L, who must have available at least one pair of unshared electrons to form the bond.EDTA (ethylenediaminetetraacetic acid) is one of the ligands that are commonly used as a standard solution and practically all metal cations can be determined.
Some important aspects of EDTA!
It is one of the most versatile complexometric titrants. It has six potential sites to form a bond with a metal ion: the four carboxyl groups and the two amino groups, each of the latter with a pair of unshared electrons. It is a remarkable reagent not only for forming chelates with all cations (chelation is a similar process to grab and hold an object with a claw), but also because these chelates are sufficiently stable in the titrations. This great stability is due to the different complexation sites that exist within the molecule, which gives it a cage-like structure that encloses the cation and isolates it from the solvent molecules.
For convenience, the free acid form of EDTA is often abbreviated as H4Y, H3Y-, H2Y2-, HY3- and Y4-, each of which predominates at a certain pH. The four dissociation constants of H4Y acid are the following:
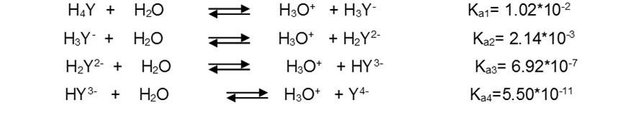
Complexometric titration with EDTA for the determination of hardness in water
What indicator can be used in EDTA degrees and how do they work?
There are about 200 compounds that can act as indicators in the titrations of metal ions with EDTA. Most of these compounds are organic dyes called metachromatic indicators that form stable complexes with metal ions. To function as an indicator in an EDTA valuation, the metal-indicator complex must have a different color from the free indicator itself. In addition, the formation constant of the metal-indicator complex must be less favorable than that of the metal-EDTA complex so that the indicator is released at the equivalence point[4].
An example!
Eriochrome black T is a typical indicator of metal ions that is used in the determination of total hardness. It behaves like a weak acid.
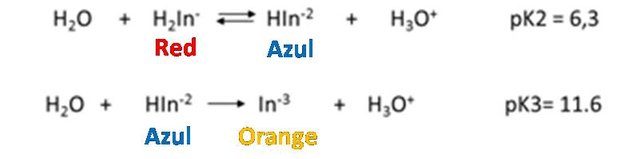
How does it work?
As it is observed, the dissociation constants of the acid are small, so it is to be expected that the solution, the predominant form of the indicator is its undissociated form of violet red H2In-. Nevertheless, the Eriochrome black T metal complexes are often also red, which is why it coincides with the color of the indicator, which makes it difficult to detect a perceptible color change at the end point of the assessment. It is for this reason that a displacement of the equilibrium towards the products must be achieved in the first dissociation of the indicator, then the HIn2- species of blue color predominates. The displacement of the equilibrium is achieved by adjusting the pH between 8 and 10 with a buffer solution. It is generally adjusted to 10 by adding a solution of ammonia-ammonium chloride. Up to the point of equivalence the indicator forms complexes with the excess metal ion, so the solution is red. With a slight excess of EDTA the solution turns blue because the indicator is released.
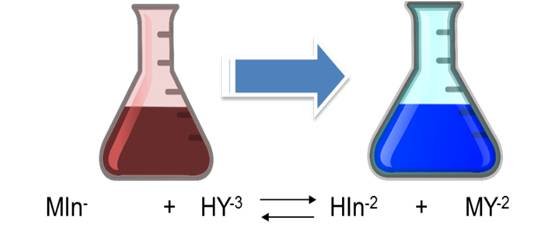
The calcium hardness is given by the amount of Ca+2 ions present in the water sample. For its determination, the pH must be adjusted between 12 and 13 so that the magnesium precipitates as Mg(OH)2 and does not intervene in the reaction. In this case, the indicator used is ammonium purpurate or murexide. At the beginning of the titration the EDTA forms a complex with the calcium that was in the solution. The complexes that form with the metals are colored, being red with calcium and yellow for the metals cobalt, nickel and copper. At the equivalence point it returns to its original color (purple) indicating the end point of the valuation.
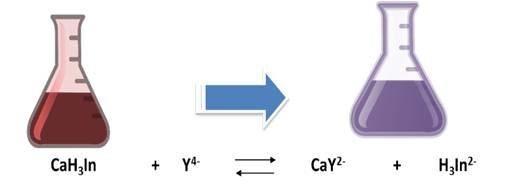
Thus, once the total hardness and calcium hardness is determined, the magnetic hardness can then be calculated.
TOTAL HARDNESS = Calcic hardness + magnetic hardness
Magnesium hardness = Total hardness - Calcic hardness
What does the result of the analysis tell us?
Once the water is analyzed it can be classified according to the content of CaCO3 present[5]:
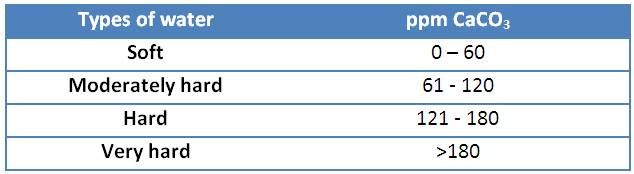
It should be noted that water can be treated by softening methods to reduce the concentration of salts.
Now we see, why it is very important to know the hardness of the water we have in our home or place of work to prevent obstructions problems and the output of soap we want.
I hope the information presented will be very useful, until the next opportunity.
References
[1]. Skoog W., Holler C. Analytical chemistry. 7ma edition.
[2]. El problema de las incrustaciones de cal y oxido.
[3] Dureza del agua.
[4]. Harvey, D. (2002). Modern analytical chemistry.
[5]. Hard water.
Hello @yusvelasquez, very interesting your post, in this case you should not use the stem-espanol label, only stemstem
Hello @carloserp2000. Ok, aware, thanks for mention it
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Thanks for having used the steemstem.io app. You got a stronger support!
Thanks for the support!!
Hi @yusvelasquez!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
many thanks friends!
Congratulations @yusvelasquez! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Congratulations @yusvelasquez! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!