Stoichiometry
In chemistry, stoichiometry (from the Greek "stoicheion" (element) and "metric" (measured) is the calculation of the quantitative relationships between reactants and products in the course of a chemical reaction.
Stoichiometry is an indispensable tool in chemistry. Problems as diverse as, for example, measuring the concentration of ozone in the atmosphere, determining the potential yield of gold from a mine and evaluating different processes to convert coal into gaseous fuels, comprise aspects of stoichiometry.
The first one to enunciate the principles of stoichiometry was Jeremias Benjamin Richter (1762-1807), in 1792. He wrote:
Stoichiometry is the science that measures the quantitative proportions or mass relationships in which the chemical elements are involved.

Scientific principle
In a chemical reaction, a modification of the substances present is observed: the reactants are modified to give rise to the products.
On a microscopic scale, the chemical reaction is a modification of the bonds between atoms, by displacements of electrons: some bonds are broken and others are formed, but the atoms involved are conserved. This is what we call the law of conservation of matter (mass), which implies the following two laws:
1.- The conservation of the number of atoms of each chemical element
2.- the conservation of the total load
The stoichiometric relationships between the quantities of reactants consumed and products formed depend directly on these conservation laws, and are determined by the (adjusted) reaction equation.
Adjust or balance a reaction
What does it mean to adjust or balance a reaction? Let's see.
A chemical equation (which is nothing more than the written representation of a chemical reaction) must reflect what actually happens before the reaction begins and at the end of the reaction and, therefore, must respect the laws of conservation of the number of atoms and the total charge.

To respect these rules, a number called a stoichiometric coefficient is placed before each chemical species, which indicates the proportion of each species involved (it can be considered as the number of molecules or atoms, or of ions or moles, that is, the amount of material that is consumed or transformed).
For example:
In the combustion reaction of methane (CH 4), it combines with molecular oxygen (O 2) in the air to form carbon dioxide (CO 2) and water (H 2 O).
The unadjusted reaction (only representing the elements that interact) will be:

This reaction is not correct, because it does not comply with the law of conservation of matter. For the hydrogen element (H), for example, there are 4 atoms in the reactants (CH 4) and only 2 in the products (H 2 O). The reaction is adjusted by introducing an appropriate stoichiometric coefficient in front of the chemical formulas of each compound.
This way, if you put a 2 in front of the H 2 O:

preservation is respected for carbon (C) and hydrogen (H), but not for oxygen (O), a situation that can be corrected by placing another 2 in front of O 2 in the reagents:

and finally, the adjusted reaction is obtained.
It says that 1 molecule of methane (CH 4) reacts with 2 molecules of molecular oxygen (O 2) to give 1 molecule of carbon dioxide (CO 2) and 2 molecules of water (H 2 O). If we verify the number of atoms we will see that on both sides of the equation there is 1 carbon atom (C), 4 hydrogen atoms (H) and 4 oxygen atoms (O). Matter (the number of atoms) has been preserved once the chemical reaction has ended.
See: PSU: Chemistry,
Question 06_2005
Question 05_2005 (Chemistry2)
Question 06_2005 (Chemistry2)
Question 13_2006
Stoichiometric coefficient
Since we mentioned it above, let's add something more about the stoichiometric coefficient.
It is the coefficient (a number) that corresponds to each chemical species (element) in a given chemical equation. In the previous example:

The coefficient of methane is 1, that of oxygen 2, that of carbon dioxide 1 and that of water 2. Stoichiometric coefficients are in principle whole numbers, although to adjust certain reactions some times fractional numbers are used. In essence what this coefficient indicates is the number of molecules of each substance.

When the stoichiometric coefficient is equal to 1, it is not written. Therefore, in the example CH 4 and CO 2 do not have any coefficient ahead.
This method of scoring to fix the stoichiometric coefficient works well when the reaction is simple. Consists of arbitrarily set a coefficient and deduct the rest making balances to the atoms involved in the initial species. If fractions appear, multiply all the coefficients by the least common multiple (mcm) of the denominators
In more complex reactions, as in the case of redox reactions, the ion-electron method is used.
It is recommended to go balancing following the order: metals, not metals, hydrogens, oxygens.
Mixture / proportions / stoichiometric conditions
When the reactants of a reaction are in quantities proportional to their stoichiometric coefficients it is said:
The mixture is stoichiometric;
The reagents are in stoichiometric proportions;
The reaction takes place under stoichiometric conditions;
The three expressions have the same meaning.
Under these conditions, if the reaction is complete, all reagents will be consumed giving the stoichiometric amounts of corresponding products.
Example
How much oxygen is needed to react with 100 grams of carbon producing carbon dioxide?
Atomic oxygen mass = 15,9994.
Atomic carbon mass = 12.0107.
The reaction is:

To form a molecule of carbon dioxide, you need one carbon atom and two oxygen atoms; or what is the same, to form one mole of carbon dioxide molecules, one mole of carbon molecules and two mole of oxygen molecules are needed.
1 mole of carbon 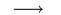 2 mole of oxygen
2 mole of oxygen
12.0107 grams of carbon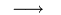 2 • 15,994 grams of oxygen
2 • 15,994 grams of oxygen
100 grams of carbon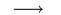 x grams of oxygen
x grams of oxygen
clearing x: 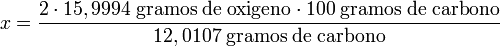
x = 266.41 grams of oxygen
realizadas las operaciones:
x = 266,41 gramos de oxígeno
See: Chemical reaction
To understand the stoichiometry one must have conceptual clarity of the following terms:
Elements -----> Atoms ------> Symbols
Compounds -----> Molecules ------> Formulas
Chemical Reactions (chemical changes) -------> Chemical Equations
Symbol is the graphic representation of an element. The symbol of an element represents not only its name, but also an atom or a prefixed number ("mol") of atoms of that element.
Formula is the graphic representation of a compound. The formula of a substance indicates its chemical composition.
Chemical equation is the graphic representation of a chemical change. A chemical reaction always involves the transformation of one or more substances into another or others; that is, there is a regrouping of atoms or ions, and other substances are formed.
https://ru.wikipedia.org/wiki/%D0%A0%D0%B8%D1%85%D1%82%D0%B5%D1%80,%D0%98%D0%B5%D1%80%D0%B5%D0%BC%D0%B8%D1%8F%D0%91%D0%B5%D0%BD%D1%8C%D1%8F%D0%BC%D0%B8%D0%BD