The debate about stratospheric ozone depletion
 Ozone layer
(License: Public Domain]: Pixabay
Ozone layer
(License: Public Domain]: Pixabay I am not here to give an explanation today but rather to receive one.
I am currently undergoing an internship with a space research and development agency in my country. Part of the program requirement is a daily two hour lecture on the fundamental concepts of Remote Sensing and Geographic Information System. It has actually been really exciting.
During the course of the lectures, I would notice that one of the tutors seemed to have some sort of problem with the existence of the ozone layer. Whenever he came up to teach and he had to make mention of the ozone layer, he would use phrases like;
science said the ozone layer is made of….
they said the ozone layer is under threat from…
He would keep on using this pattern, if not for anything, at least just to avoid any direct attribution to the ozone layer’s existence. This kept going on and on because the nature of the discussions he had to have with us obliged him to always make reference to the ozone layer.
One day I had to innocently ask him the reason for the attitude I had observed about him concerning the ozone layer. He responded by saying he does not believe in the existence of the ozone layer.
I instantly knew that things were about to get interesting because the dialogue that was about to ensue between the teacher and his student had aroused the interest of the other students; some of whom were probably getting bored of the lecture in the first place.
I have always been taught that the ozone layer is an atmospheric blanket that protects our dear planet Earth from getting roasted by the sun (with us in it), but as one who likes to approach those kind of situations with an open mind, and understanding how highly intelligent this young man is, I politely asked for his reason, which he also politely gave by saying;
Science believes in facts, that is; what can be seen… or, better put; what can be proven to be seen.
I wore on my face a quizzical look. If you are reading this, I think you know what I’m trying to get at now. You can’t physically see oxygen too, you know? For someone I and the other students all know to be extremely knowledgeable about science, I wasn’t as much confused as I was disappointed.
But first you said “science said…”, now you say you don’t believe in what “science said” because you are a scientist
That was the statement i threw back at him. I think you know at this point, that the dialogue wasn’t going to end in a very satisfying manner.
So I sought out on my own to know if there were other scientists with which he shared the same view and why they hold such view, and whether they actually have any valuable argument.
But first, I want you to disregard what I said earlier. I actually have some interesting explanation to do first about the ozone layer, starting with where it can be found; a review of elementary geography.
A few blocks down the street...
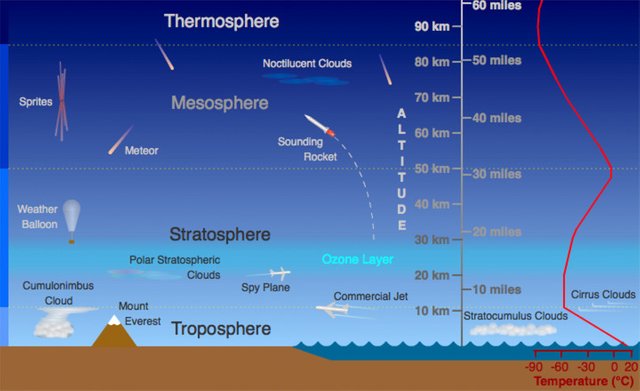 Atmospheric Layers
(License: CC BY-SA 4.0 ]: Wikicommons
Atmospheric Layers
(License: CC BY-SA 4.0 ]: Wikicommons Elements within the Earth’s system are grouped into four major categories. These include; Water, living things, land and air. They are interconnected to each other and define the structure for which different components within the earth’s system relate with one another.
Scientifically, they are known as the Hydrosphere, Biosphere, Lithosphere and Atmosphere. These names were derived from the Greek names; hydro (for water), bio (life), litho (stone), and atmo (vapour).
The Ozone layer is situated somewhere in the atmosphere. The atmosphere houses the earth’s gases which are collectively known as Air; 79 percent of which is Nitrogen, Oxygen – 20.946 percent, with carbon dioxide, argon, and other trace gases making up the remaining meager 0.054 percent.
Based on temperature, the atmosphere is further subdivided into four principal layers. These are the troposphere, stratosphere, mesosphere and thermosphere.
The troposphere
This is the part of atmosphere closest to the earth where we live in. It is the lowest part of the atmosphere. The clouds and almost everything we know as weather can be found here because this is where most atmospheric water vapour are housed. It contains a very large proportion (75% - 80%) of all the air in the atmosphere. Its height ranges from about 9 km (at the poles) to 18 km (at the equators) with an average height of 12 km. The lowest part of the troposphere is known as the boundary layer while the topmost part is known as the tropopause.
It is pertinent to note that within the troposphere, an increase in height is accompanied by a decrease in temperature (6.5 degree Celsius per kilometer) and pressure. I believe Mountain climbers will agree with me on this.
The Stratosphere
The stratosphere starts just above the tropopause to about 50 km from the surface of the Earth. Here, in contrast to the troposphere, temperature increases with altitude, from a nearly constant temperature within its lower portion to an increasing temperature in the upper portion.
The Ozone layer can be found in the stratosphere. This is where most (about 90%) of the ozone concentrations in the atmosphere is housed. The ozone layer is situated in the upper portion of the stratosphere and is the reason why temperature increases with altitude in that portion of the atmosphere. This is caused by the absorption of ultraviolet rays from the sun by the layer to prevent them from getting to the earth’s surface.
Most conventional aviation activity takes place in the troposphere, and it is the only layer that can be accessed by propeller-driven aircraft. The stratosphere is the highest layer that can be accessed by jet-powered aircraft. Source
Going higher, the mesosphere follows the stratosphere before getting to the thermosphere. The other spheres beyond the thermosphere include the exosphere and magnetosphere. Since we have already found the location of the stratosphere, there would be no need of going further for the purposes of this study. Besides, you wouldn’t want to travel as far as to the magnetosphere for any reason. Would you? :p
However, you could read about these other layers of the earth’s atmosphere here and here.
Let’s meet Ozone and the Ozone layer
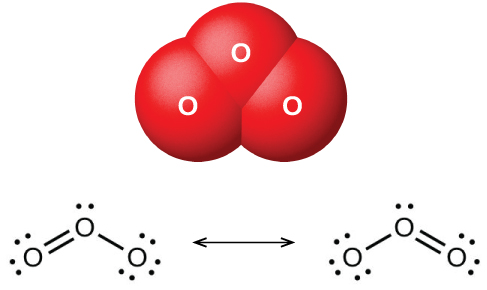 Ozone molecule
(License: CC BY-SA 4.0, Author: OpenStax]: Wikicommons
Ozone molecule
(License: CC BY-SA 4.0, Author: OpenStax]: Wikicommons There are various albeit similar definitions and descriptions of the ozone layer. It is generally known as the layer of ozone concentrations that is located in the upper part of the stratosphere. The description below gives a comprehensive view of the ozone layer;
Ozone is a gas that is naturally present in our atmosphere. Each ozone molecule contains three atoms of oxygen and is denoted chemically as O3. Ozone is found primarily in two regions of the atmosphere. About 10% of atmospheric ozone is in the troposphere, the region closest to Earth (from the surface to about 10–16 kilometers (6–10 miles). The remaining ozone (about 90%) resides in the stratosphere between the top of the troposphere and about 50 kilometers (31 miles) altitude. The large amount of ozone in the stratosphere is often referred to as the “ozone layer” Source
An ozone molecule contains 3 oxygen atoms. The name Ozone is gotten from a Greek word “ozein” which means “to smell”, because of its pungent odour. Industrially, it is produced by electrical discharges for the purpose of purification of air and water. The little amount of ozone present in the troposphere is produced from chemical reactions between naturally occurring gases such as Nitrogen and also gases such as hydrocarbons that get to the atmosphere from pollution sources.
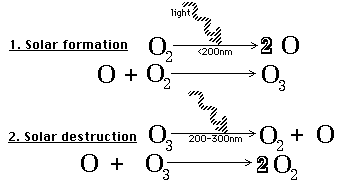 Ozone formation and destruction
(License: Public Domain]: Wikicommons
Ozone formation and destruction
(License: Public Domain]: Wikicommons Ozone is formed naturally in the stratosphere when ultraviolet radiation from the sun reacts chemically with oxygen molecules within that layer. The ultraviolet rays strikes an oxygen molecule to produce two oxygen atoms. A single oxygen is known as atomic oxygen. Atomic oxygen (O) then reacts with another oxygen molecule (O2) to produce an ozone molecule (O3). Concentrations of ozone molecules in the atmosphere constitute the ozone layer, absorbing these ultraviolet radiation from the sun. These ultraviolet rays in turn split the ozone molecule back into an oxygen molecule and an atomic oxygen.
Depletion of the ozone layer
The amount of ozone in the stratosphere remains constant as long as the rate of ozone production equals the rate of its destruction. Just like every other balancing process that occurs in nature. However, this seems to no longer be the case. It’s not a fresh news just as much as it isn’t good news that man (the usual culprit) has interfered with that natural balance of the ozone layer.
 Air pollution
(License: Public Domain]: Pixabay
Air pollution
(License: Public Domain]: Pixabay Halogen source gases such Chlorofluorocarbons (CFCs), Carbon tetrachloride (CCl4), Halon-1211 (CBrClF2), Halon-1301 (CBrF3) are emitted from the earth’s surface into the troposphere from anthropogenic industrial and commercial activities. This accompanies the release of natural source gases like Methyl chloride (CH3Cl) and Methyl bromide (CH3Br) from coastal salt marshes and other natural phenomenon.
Let’s focus on the chlorine related gases for the purposes of this explanation. When these chemical gases get into the troposphere, they do not react with other chemicals because of their relative stability. So what happens is that they accumulate and are carried into the stratosphere by means of natural air motions. In the stratosphere, interaction with ultraviolet rays from the sun causes them to split into chlorine atoms. The chlorine atoms then form chlorinemonoxide (CLO) as they displace and combine with one oxygen atom from ozone molecules.
These CLO then react with free atomic oxygen which displaces the oxygen atom in the CLO, thereby releasing chlorine atom to roam and displace more ozone molecules.
These two reactions happen over and over again so that a single atom of chlorine, acting as a catalyst, destroys many molecules (about 100,000) of ozone. Source
The effect of this is as straightforward as you can think it to be; since the major role of ozone molecules in the stratosphere is to absorb dangerous ultraviolet rays and prevent them from getting to the earth’s surface, a depletion of ozone in that upper atmosphere allows for those radiation to get to the earth’s surface. This phenomenon is best described with the leaky bucket analogy below;
This is like a leaky bucket: If you pour water into the bucket at the same rate that it’s leaking out, the level of water in the bucket will stay the same.
In the 1970’s, scientists suspected that reactions involving man-made chlorine-containing compounds could upset this balance leading to lower levels of ozone in the stratosphere. Think again of the “leaky bucket.” Putting additional ozone-destroying compounds into the atmosphere is like increasing the size of the holes in our “bucket” of ozone. The larger holes cause ozone to leak out at a faster rate than ozone is being created. Consequently, the level of ozone protecting us from ultraviolet radiation decreases. The ozone destroyed by man-made emissions is comparable or more than the amount destroyed by natural processes. Source
Although it would be interesting to point out that holes are not literally created in the ozone layer but a thinning of the layer is what actually occurs. This has been going on since the mid-1970s. The resulting consequences are an increase in global temperatures, melting of Ice in the Antarctica (The ozone hole phenomenon), threat of skin cancer and cataract, ad infinitum. These are collectively known as the effects of global warming.
The Ozone depletion Skeptics
There are however arguments against the significant existence of an ozone depletion in the stratosphere. This arguments arose as a result of government’s attempt to place a regulation on the production of CFCs.
DuPont, which made 1/4 of the world's CFCs, spent millions of dollars running full-page newspaper advertisements defending CFCs in 1975, claiming there was no proof that CFCs were harming the ozone layer. Chairman Scorer of DuPont commented that the ozone depletion theory was "a science fiction tale...a load of rubbish...utter nonsense." (Chemical Week, 16 July 1975). Source
Here are some of the points imployed to back up their argument;
i. The sunspot cycle. Sunspots are dark regions on the sun's surface that have relatively lower temperatures to the regions around them. The sunspot cycle describes the rise and fall in the amount of these regions over the years. There is insufficient information to prove that ozone depletion, if any, is not chiefly due to this natural occurrence.
scientists have little certainty about how much ozone is actually being degraded by CFC's. Source
ii. There is no sufficient experimentation to accurately understand the how aerosols and nitrogen-oxides (NOs) interact with ozone and chlorine atoms in the stratosphere.
iii. The negative effects of placing a ban on CFCs production are a lot more to the benefits attached to it because these are the major sources of refrigerants in refrigerators and air-conditioners.
Refitting and replacing industrial equipment that cannot accept CFC alternatives is estimated at $130 billion in the US alone! “The unfortunate outcome (if the CFC-ozone link proves false) may be the unconscionable waste of resources, a loss of public trust, and a real setback for the environmental effort.'' Source
Well, however accurate these points may seem to be to anyone, it is evident that the skeptics that make such arguments against the significance of the ozone layer depletion are clearly propelled by personal selfish interests of certain CFC manufacturing companies.
As for the tutor in the agency where I am interning, I would want to believe he was actually referring to not believing in the ozone-layer depletion and not the ozone layer existence as he may have appeared to make it seem. That is the only logical explanation I could use to convince myself about his stance. Even at that, I do not think he owns a CFC manufacturing company, so I'm still searching for answers.
References
Twenty Questions and Answers about the Ozone Layer: 2010 Update
Whoa, I am shocked to read that what seems to be a bright lecturer does not believe in the depletion of the zone layer or the ozone layer as a whole for that matter.
Also his definition of science is rather unscientific, with such a view quantum physics would not be science...
Anyways great article, before reading this I understood the concept but not the details so thanks for that!
PS: Is it possible to estimate the rhythm of depletion? Even if it is, I think most people that do not believe in the depletion and global warming will not change their minds... Especially with leaders like Trump :
This video really cracked me up 😂😂
I really like trump but he just sounds dumb at times.
That aside, talking about the teacher.. I'm amazed myself. It would be really difficult to believe that he is the same person that breaks down all that scientific stuff for us in class.
Exactly. It really baffles me cos he's at least master's degree holder.
About estimating the depletion rhythm.. I've not read much about that but it might interest you to know that halogens don't live long in the stratosphere. The reason why the depletion seems to be increasing is because we keep launching them into the atmosphere to replace their old friends. So its currently a continous cycle.
Whoa is this man really this ignorant ?
Hi @writeit!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
the mystery of the ozone layer!
its quite funny some people do not believe the ozone layer exists...just funny