Electrochemical surface area of Pt Nanostructures
Introduction
One of the characteristics that have been useful in the predicting applications of nanostructures and nanomaterials is the surface area. This is because, the smaller the size of nanomaterials, the higher their surface area, which makes it suitable in wider applications like catalysis, sensor and medical devices.
Electrochemical analysis has been used in determining the surface area of nanomaterials using the method called cyclic voltammetry. Cyclic voltammetry is a method used in electrochemistry to measure the current that is generated in an electrochemical cell when a certain range of potential is passed through it [1, 2].
Experimental methods
This includes the set up of an electrochemical cell
An electrochemical cell, contains
- A working electrode: Glassy carbon electrode, gold macroelectrode can be used.
- Counter electrode: platinum mesh,
- Reference electrode: Saturated calomel electrode (SCE), Mercury sulfate reference electrode (MSRE). and
- Electrolyte: Sulfuric acid
The below description is an excerpt from some research I have done in an electrochemistry laboratory.
Measurement
The analysis was done using Cyclic voltammetry in a Faraday cage. This was carried out using a three-electrode system in an optimized electrochemical condition.
Results
Below are shown a typical example of the cyclic voltammogram recorded using my in-lab prepared platinum nanostructures.
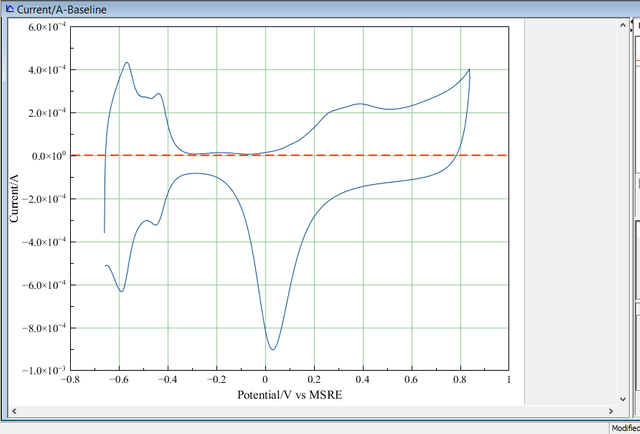
The voltammogram was recorded in deoxygenated 0.5 M sulfuric acid, against MSRE at 50 mV. The data obtained was plotted using a software called MagicPlot Student 2.7.2 [3]. Origin 8 or any version can also be used.
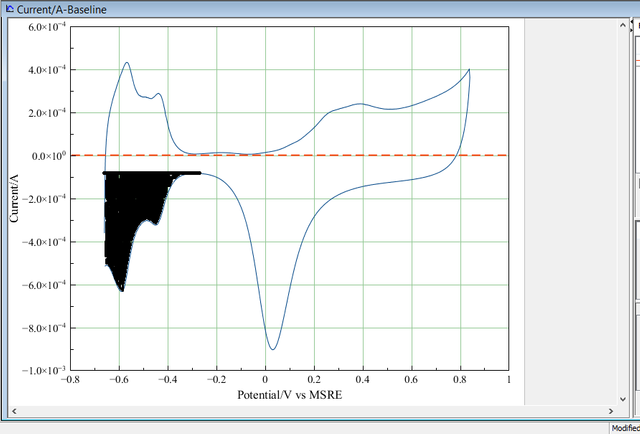
To calculate the surface area of the Platinum nanostructure, the portion relating to hydrogen adsorption is integrated(the shaded area). Using MagicPlot Student 2.7.2 , the shaded area has been integrated and shown below
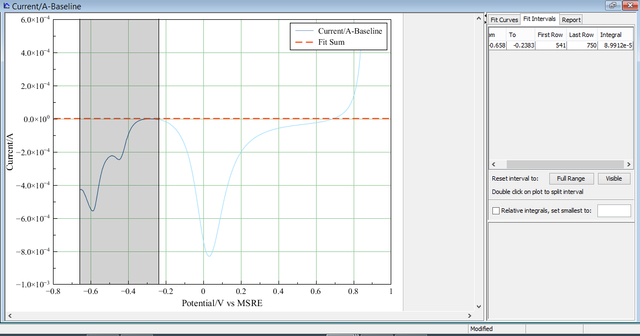
The right side of the picture, have been isolated.
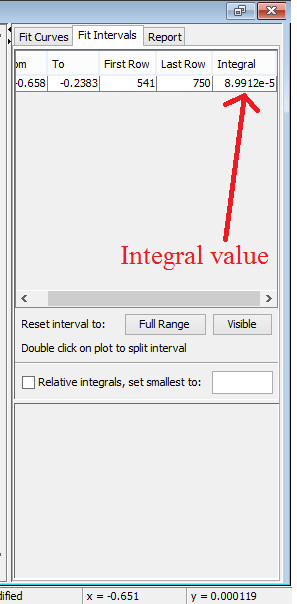
The integral value will then be used in calculating the surface area using the formula.
The formula mostly used for ECSA is
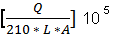
This formula is simply
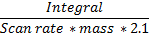
The units of
- scan rate is Volt/second,
- mass is gram, and
- the constant is C/m2
From the picture above, the integral and scan rate is known, 2.1 C/m2 is a constant which refers to the electrical charge associated with the monolayer adsorption of hydrogen [4].
The 2.1 C/m2 is specific with Platinum, other metals have individual unique constant value.
The mass is obtained by using an instrumental method which is Inductively coupled plasma -optical emission spectroscopy (ICP-OES). This is used to determine the actual mass of platinum nanomaterials involved in the hydrogen adsorption. This is made possible by the Cyclic voltammetry.
Since this is excerpt of my research, from my experimental result, I got the mass to be 2.0 * 10-6 g
Therefore I will substitute this into the equation above
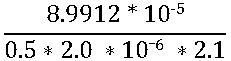
This gives 42.9 m2/g.
Summary
- The calculated electrochemical surface area of the synthesized platinum nanostructure is 42.9 m2/g.
- This procedure can assist anyone new into electrochemistry to calculate the electrochemical surface are (ECSA).
- This helps in determining the application that this nanomaterial can be used for. However, this high surface area would mean efficient catalytic ability of the platinum nanostructure in low-temperature fuel cells.
- This post is to guide people that are into Electrochemistry and nanomaterials synthesis.
Thanks.
All the pictures are mine.
References
[1].https://chem.libretexts.org/Core/Analytical_Chemistry/Instrumental_Analysis/Cyclic_Voltammetry
[2]. Charoen-amornkitt, Patcharawat, Takahiro Suzuki, and Shohji Tsushima. "Ohmic resistance and constant phase element effects on cyclic voltammograms using a combined model of mass transport and equivalent circuits." Electrochimica Acta258 (2017): 433-441.
[3]. https://magicplot.com/downloads.php
[4]. Sheppard SA., Campbell SA., Smith JR, Lloyd GW, Walsh FC and Ralph TR, Analyst, 1998, 123, 1923–1929
Hmmmm, electrochemistry, one of the most interesting part of chemistry, i really loved it then in school.
Hahahaha...your post reminded me that i was once, rather still a chemist. Just let flying take over. Someday i will comeback to it. I loved teaching chemistry back then.
Trying to understand those curves almost gave me a headache. I guess it's because it's still early but well detailed post bro and steem on
Well, it more of electrochemistry. Those curves are pretty explanation of chemical response of nanostructures.
This is the reason why I prefer biological sciences. I don't have to do serious calculations!. Anyways, it seems you know your onions though as your post is quite explanatory.
Thanks. You are right. Calculations help scientists in all fields, anyway.
Glad to see someone loves calculations cos I hate it. Nice post
Yeah, we cannot avoid that in science, especially in Physical Chemistry.
This post has received a 0.39 % upvote from @drotto thanks to: @steemstem-bot.
Well done! This post has received a 7.69 % upvote from @litasio thanks to: @steemstem-bot. Whoop!
If you would like to delegate to the @LitasIO you can do so by clicking on the following link: 10SP