Conditions Influencing rate of Photocatalytic Degradation of pesticides and other organic Pollutants in water bodies.
Good day everyone. In my last post, on the use of photo catalyst to degrade pesticides, i stopped at where I gave the list of some factors considered to affect the efficiency of photo catalyst in degrading the organic pesticides to non toxic components. In this post, I will go deep into each of the factors, so sit down and enjoy the post while it last.
Loading of catalyst for the degradation. Measured volume of TiO2 to the substrate should be taken into consideration. The substrate here means the pesticides. When the amount of the catalyst applied is not given adequate consideration and it is more than the required, desired result may not be gotten.
If for instance, the amount of TiO2 supplied on the surface of the substrate is beyond a specified amount, the amount of light adsorbed will decrease, this is due to the fact that the extra catalyst on the substrate will provide a screening effect (shielding) on the surface, such that where the extra catalyst cover will not be exposed to incident light. This then result to less photo catalytic degradation of the Organic Pesticides/waste.
Another point we will look at is the pH of the environment where photocatalysis is to take place
The treatment of water polluted with either organic pesticides or any other Organic matter with photo catalytic process required controlled hydrogen ion concentration (pH).
TiOH + H+ -----> TiOH2+
TiOH + OH-----> TiO- + H2O
At lower hydrogen ion concentration ( pH), TiO2 optimum oxidizing strength is attained. On the contrary, too much of hydrogen ion which means the pH is low leads to a reduction in the efficiency of the photo catalytic process.
Photocatalyst shape and magnitude :The shape and magnitude of photocatalyst in degradation of Organic Pesticides is highly needed. This become imperative because the nature of surface area of catalyst plays a direct role in it degradation of Organic compounds. The kinetic Studies of the catalyst in relation with the organic pesticides shows that the available surface of the catalyst for the incident photon determine the reaction rate. The reaction only takes place on the catalyst surface open for photons absorbtion.
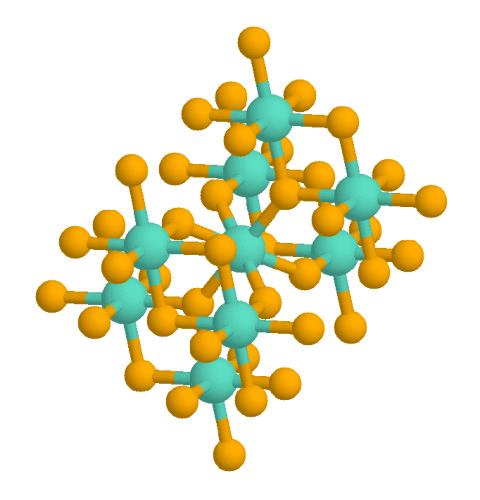
Structure of Rutile Titanium dioxide:Own work, Public Domain
Temperature under which the reaction is taking place is also important : Photocatalytic process is optimized when the temperature range of the process is within the limit that supports the condition under which the catalyst can work. When the reaction temperature increase, it correspond to increase in the reaction rate.
On the contrary, the temperature should still be maintained, because if it rise above certain level like 80°C , it can be deleterious to the reaction, as this could lead to the charge carriers on the catalyst being recombined and thereby makes the absorbtion onto the surface of the catalyst by the organic pesticides to be compromised. In the temperate regions, photocatalytic process can be greatly achieved because of the favourable weather condition compared to the frigid regions which mostly have very low temperature.
Pollutants Concentration and nature.: The nature of the organic pollutants/ pesticides to be degraded must be given adequate attention, because it influence the degrading power of the catalyst. When the organic pesticides is washed down to water body, it's unlikely that the pesticides is the only compound present, so the combination of the pesticides concentration and it nature couple with the existing matters in the water body greatly affects the reaction rate.
The surface of the Titanium dioxide become highly saturated when it's own concentration in the polluted water body is less than the pollutants. This then results to reduction in efficiency of the catalyst in it's photocatalytic action.
The structure of the compound targeted for degradation also determine the rate of the reaction and the efficiency of the catalyst.For instance, some compounds will have to breakdown to several intermidiate before finally getting down to it's minerals, where's some may just breakdown directly to it's minerals.
If the pesticides compound is the one that attached it self to the surface of the catalyst, that makes the degradation an easy job. The substituent group on the compound to be break down is also part of this factor been discussed. If the substrate (organic pesticides) possess an electron withdrawing properties [An electron withdrawing molecule or atom is the one that when present in a compound tends to attract or have high affinity for electron], with this property, it get itself glued to the catalyst and are with that easily oxidized when compared to compound containing electron donators.
Presence of Inorganic Ions: The presence of Inorganic Ions in the water body polluted with pesticides can largely affect the photocatalyst efficiency in degrading the organic pesticides. This is due to the fact that these ions can themselves adsorbed to the surface of the photocatalyst.
There are two forms of applying TiO2, which includes the slurry form and the fixed-bed configuration. Research had shown that at both forms, some ion such as Copper, Iron, Phosphate can deactivate the photocatalyst if they are present at a certain amount, whereas, some other ions such as Zinc, Calcium, Magnesium, has little impact on the photocatalyst degradation of organic compound, this could be attributed to the high oxidizing state of these cations such that they possess low inhibitory tendency towards the activities of the catalyst.
Some other Inorganic anions like sulphate, chloride, nitrates too also have inhibitory tendency towards the surface of the photo catalyst. Salt been formed makes the interaction between the catalyst surface and the pollutants to be reduced. The anions do not only cleaved to the surface of the catalyst, but they also hunt for the hole on the surface of the catalyst and scavenge for the hydroxyl radicals form during the cause of the catalytic process such that the mineralization of the pollutants is incomplete. Examples of anions responsible for this effect are Cl-, PO42- and CO32-.
However, in other to prevent this phenomenal, the polluted water body could be treated using an ion exchange resins
which can exchange the unwanted ions from the water so that the photo catalytic degradation process can take place without much hindrance. Apart from the ions mentioned, there may still be several ions both cations and anions. The type of ion present depends on the environmental activities taking place. For instance, the ions may come in through to the environment (water majorly) through the use of fertilizer or other human activities.
Summary
Measured volume of TiO2 to the substrate should be taken into consideration. When the amount of the catalyst applied is not given adequate consideration and it is more than the required, desired result may not be gotten.
The treatment of water polluted with either organic pesticides or any other Organic matter with photo catalytic process required controlled hydrogen ion concentration (pH).
The shape and magnitude of photocatalyst in degradation of Organic Pesticides is highly needed.
Photocatalytic process is optimized when the temperature range of the process is within the limit that supports the condition under which the catalyst can work.
The presence of Inorganic Ions in the water body polluted with pesticides can largely affect the photocatalyst efficiency .
The nature of the organic pollutants/ pesticides to be degraded must be given adequate attention, because it influence the degrading power of the catalyst.
Thanks for stopping by to read this write up.If you learn anything from, please don't hesitate to leave a comment behind. You can join us on @steemstem discord server by clicking on DISCORD . Thanks for staying tuned.
REFERENCES
Chong M.N., Jin B., Chow C.W.K Saint C. Recent Developments in Photocatalytic Water treatment Technology. A review. Water Resources 2010(44) 2997-3027
Gaya U.I., Abdullah A.H. Heterogeneous Photocatalytic Degradation of Organic contaminant over Titanium dioxide. A Review of Fundamental Progress and problems. Journal of PhotoChemistry and Photobiology .C PhotoChemistry Reviews 2008(9) 1-12
Bahnemann D. Photocatalytic Water Treatment: Solar Energy Application. Chemical Engineering Journal 2004(102)283-290
Intechopen.com/books/organic-pollutants-monitoring-risk-and-treatment



This post reminds me of a work I authored last week about accidental discovery of bio enzyme that can actually degrade plastic, especially the type used in making bottles.
Very informative post on conditions that affect the rate at which photocatalytic breakdown of pesticides in water bodies. Kudos to you.
@eurogee of @euronation community
Thanks for stopping by @eurogee. Science is evolving daily. We must save ourselves and the planet from the danger we exposed it to.
✌️
Great chemist. keep up the good work of educating us.
Thanks @saintgentle for stopping by and for the comment.
For the degradation of any Organic pollutant (such as Pesticides) to occur, attention must be given to volume/amount of catalyst, pH of the environment, shape and magnitude of photocatalyst, temperature of the reaction, presence of Inorganic Ions and the nature of the organic pollutants/ pesticides to be degraded.
Kudos for supplying such detailed explanations
Resteemed
Thanks doc for stopping by. Those factors must be considered for effective degradation of pesticides especially in water bodies. Thanks @oluwambe
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Steepup from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
kudos @steepup, very enlightening.
Thanks @conas, for stopping by. We are meant to enlighten the world.
Good post @steepup. Thanks for educating us.