MICROSCOPIC SLIDE PREPARATION TECHNIQUE
Hi steemians, I really appreciate your effort for taking your time reading through my blog always, just that we have to share our experience to each other but today, I want us robs mind together on microscopic slide preparation techniques, I was preparing the slide today in the laboratory and I asked my students why there's a need for slide preparation in the laboratory, 90 percent of the students had no idea.
Microscopic slide preparation is a method used to preserve stain of different types in the laboratory either permanent or temporary for a long or short period of time.
Microscopic slide preparation falls into two categories; There is temporary slide preparation which has to do with slides prepared for short time observation. For instance, microscopic slides prepared in the hospital for malaria parasite (MP), Also, there is permanent slides preparation which involves slide prepared for long-time observation. Examples are various slides, prepared and kept in schools for practical use.
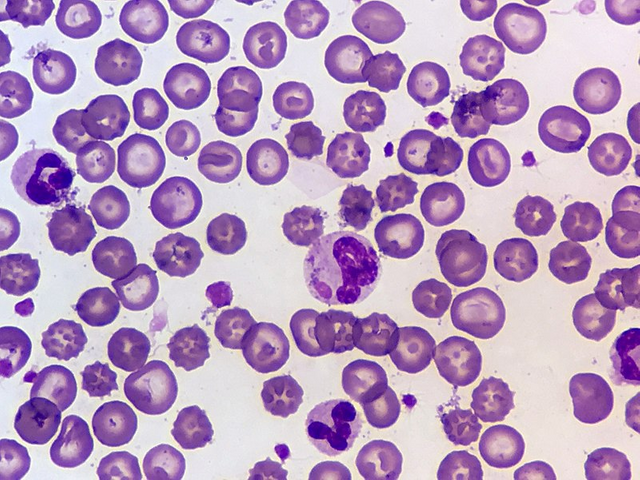
By Lance Wheeler - Own work, CC BY 4.0,
STEPS INVOLVED IN PERMANENT SLIDE PREPARATION
FIXATION: This is a preventive method to prevent the material from decaying. It involves dipping the material or tissue inside fixatives chemical for some time before further processes.
The common fixatives used for this process include; Acetic acid which can be used as Ascetic alcohol. Acetic alcohol is called Carnoy’s fluid. It is used for histological mitotic cell division. Formalin, It is used for plant and animal tissue. Bouin’s Fluid is also good for plant and animal tissue. Chromic Acetic Acid is only used for plant tissues. Formalin Acetic Alcohol is used for plant tissues.
Fixation is very important for the following reasons; It kills the cells and fixes the cell so that their physiological feature is not altered, It prepares the tissues for subsequent staining treatment, It hardens the tissue or organ for sectioning, It also precipitate protein and so helps the cells to retain its original structure.
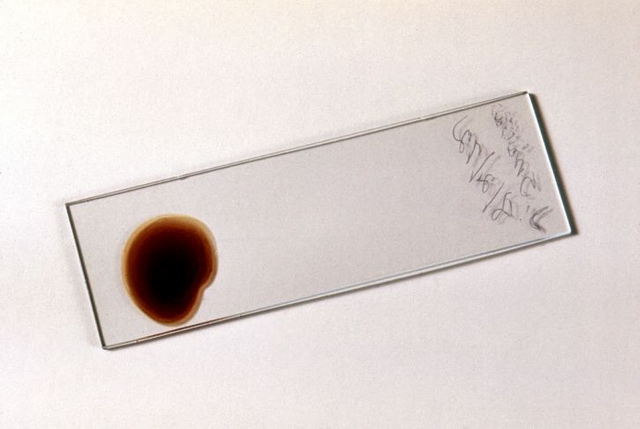
prepared slide for thick blood smear
DEHYDRATION
After fixation, we move on to dehydrate the material. This involves washing of the material in graduated alcohol in the increasing order (i.e, from 20% to 30% to 40% to 50% and 70%). CLEARING: This is a remover of alcohol which can react with wax. it is done by transferring the materials into xylol.
Clearing Agents: For temporary preparation, isotonic saline is commonly used for animal tissues and 2,2,2- trichloroethanediol (chloralhydrate) for animal tissues. A number of clearing agents are available for permanent prepareations, of which those most commonly used include xylene, clove oil, cedarwood oil, terpineol, Berlese’s fluid and Amann’s medium.
Xylene has the disadvantage that it causes some shrinking and hardening of tissues. It also forms a white emulsion if there is the slightest trace of water remaining in a specimen which has been incompletely dehydrated. 5g phenol dissolved in every 100cm xylene (dimethylbenzene) will reduce the occurrence of this emulsion.
Clove oil is frequently used for plant tissues, but it does render them brittle and remove some stains such as haematoxylin and safranin.it is also necessary to remove all traces of clove oil with xylene before mounting in Canada balsam or synthetic resin. Cedarwood oil has in the past been considered one of the finest clearing agent for animal tissues. It is slower in its action than xylene but causes less shrinkage or hardening. Like clove oil is must be removed with xylene before mounting in Canada balsam.
However, its great disadvantages are that it is very expensive. Terpineol may be used instead of cedarwood oil and is very much cheaper. Berlese’s fluid is frequently used for clearing small whole mounts of delicate arthropods. Amann’s medium is used for clearing delicate algae, fungi and small nematodes. Embedding (block making) is a process of placing the material inside molten wax in the mould. Before it solidified mark should be made to differentiate either head or tail of the material.
Sectioning
The process of sectioning is done by the use of a microtome. First, trim the base of the block by cutting. Melt the trimmed end of the block using hot forceps and fix it on the wood block of a microtome. To ensure proper fixing of the block set the parallel side of it to the parallel side of the microtome razor blade. Then set the microtome to the required micron.
To ensure that the block has been properly fixing and microtome well set the following are things to notice. A Section must form a strip. If it does not form strip it will form curling and that shows the blade is blunt. The Section must form a ribbon, If it does not it means that the block is not properly trimmed to be parallel to the blade.
pixnio.com/free-images/science/microscopy-images/malaria-plasmodium
Smearing: for semi-fluid tissues like blood which cannot be sectioned, they can be placed on one side and the edge of another slide can be used to spread it and Squashing involves the pressing of soft tissue on a slide with a coverslip to spread it before staining. Staining: the first step in staining paraffin section is the section removal of wax either by mounting on a hot plate or dip in xylol to remove the molten max. then hydrate to soften the tissue.
METHODS OF STAINING INCLUDE
Progressive staining: this is the process in which the section is left in the stain until it reaches the required dept of colour. Regressive staining: this is the process in which the section is intentionally over stained and then differentiated or decolourised to required colour. Double staining: this is the process in which section is stained with two different stains, one at a time. The two stain are normally differentiated with acid alcohol and hot water.
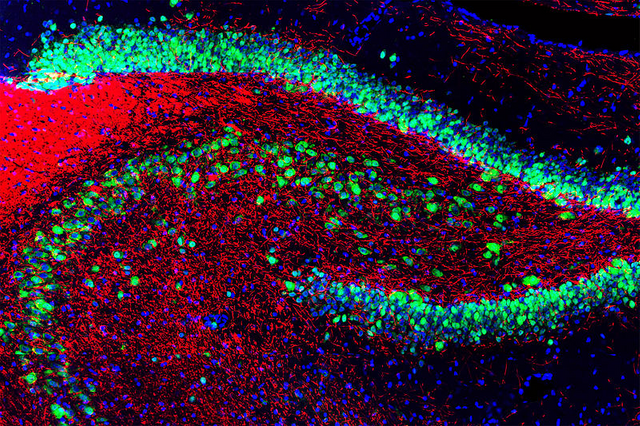
By GerryShaw - Using a confocal microscope and fluoresent antibody stainingPreviously published: 2015-11-10, CC BY-SA 3.0,
TYPES OF STAIN
Most stains are coloured organic dyes which are soluble in water or alcohol or both. They have been classified in various ways of which the most useful is as follows; Basic or nuclear stains consists of a coloured organic base in combination with an acetate, sulphate or chloride radical. Such stains have an affinity for the nuclei of cells owing to the presence there of nucleic acids. Examples are safranin and haematoxylin.
Acidic or cytoplasmic stains consist of a metallic base combined with a coloured organic radical. The cytoplasm of most cells tends to be basic and thus to have an affinity for acidic stains. It is therefore described as acidophil. Eosin is perhaps the most familiar example of an acidic or cytoplasmic stain. Neutral stains are normally a mixture of acidic and basic dyes.
Certain dyes will not become attached to cellular material unless a mordant used. It appears that the mordant becomes attached to the tissue to form a complex compound which can then unite with the dye to form an insoluble coloured compound. For example, iron alum (ferric chloride) is used as a mordant for haematoxylin. Another example is iodine solution.
References
Congratulations @semilore! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your Board of Honor.
To support your work, I also upvoted your post!
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP@semilore, this is nice post
@steemitboard, This is another achievement for @semilore, keep on .
Hi @semilore!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
@semilore, it's such a nice post. Very informative and detailed.