INDUSTRI ALKOHOL (ETANOL)
I. the history of ethanol has been used human since prehistoric times as material drunks in alcoholic beverages. Residues found on the legacy of ceramic old 9000 year from China the northern part of shows that alcoholic beverages has been used by human the prehistoric of the Neolithic. A mixture of (bio) ethanol approaching kemrunian first found by chemist Muslim develop a process distillation during the Caliph abbasid with researchers famous time it is Jabir IBN hayyan (geber), Inter alia-Kindi (alkindus) and Inter alia-Razi (rhazes). Note compiled by Jabir IBN hayyan (721-815) mentioned that the steam from wine boiling flammable. Inter alia-Kindi (801-873) firmly describes the distillation of wine. While (bio) absolute ethanol obtained in the year 1796 by Johann Tobias lowitz, using the distillation filter arang.antoine lavoisier illustrate that (bio) ethanol is compounds formed from carbon, hydrogen and oxygen. In 1808 Nicolas-théodore de saussure can determine the chemical formula ethanol. Fifty years later (1858), Archibald Scott couper publish the formula up ethanol. Thus ethanol is one of chemical first found formula bangunnya. Ethanol first created of synthetic on know 1829 in the UK by Henry hennel and s.g.serullas in France. Michael Faraday make ethanol by using the hydration acid catalyst in ethylene in 1982 that is used in the process of ethanol production synthetic to date. In 1840 ethanol be fuel light in the United States, in the 1880s Henry Ford cars quadrycycle and since 1908 Ford model T have been able to use (bio) ethanol as fuel. But in the year 1920an fuel from Petroleum the price is cheaper has been a dominant cause ethanol less get attention. Lately, with rising oil prices, bioethanol back to get the attention and has become an alternative energy continue to be developed. II. The definition of ethanol, also known ethyl alcohol, pure alcohol, absolute alcohol, or alcohol only, is a kind of fluid volatile, flammable, colorless, and is the alcohol most commonly used in everyday life. This compound is a drug psychoactive and can be found in alcoholic beverages and thermometer modern. Ethanol is one of recreational drug most old. Ethanol included in alcohol chain single, with chemical formula c2h5oh and empirical formula c2h6o. He is isomers constitutional of dimethyl ether. Ethanol often abbreviated be etoh, with a "@" stands group ethyl (c2h5). Ethanol widely used as solvent a variety of chemicals are intended for consumption and usability humans. For example is the perfume, taste, food coloring, and drugs. In chemistry, ethanol is solvent important as well as stock feed for the synthesis of chemical other. In the history of ethanol has long been used as fuel. Ethanol is compounds that there is no freely in nature. This substance is a class of alcohol regular or alcohol primary made of glucose or type of sugar others with a way fermentation. The use of alcohol include: * as a drink * as chemicals and solvent * as Motor fuel * used in the field of pharmaceutical alcohol as liquor is divided into 2 types, namely: * drink not distilled, that is drink contains only alcohol at most 12%, example beer and wine. * Drink distilled, that alcohol approximately 55%, example whiskey, wine, cognac to alcohol use as fuel and the purposes of the pharmacy and industry not drunk, then ethanol made not terminum by given methanol and Dye (denaturation alcohol), for example alcohol used as spirtus fuel. III. Characteristics of the material and product properties: § ethanol is a liquid Crystal clear and can be mixed with water in all of comparison (is missible) § can dissolve organic compounds of raw materials to produce ethanol by means of fermentation can be in the production of 3 kinds of carbohydrates, namely: * materials sugar or also known sustansi sakharin, it's sweet such as sugar cane, sugar beet, molasses (drops), various sari fruits and others. * Material containing starch, for example: grain, corn, wheat, potato sorghum, malt, barley, cassava and others. * Materials Baha containing cellulose, for example: Wood, liquid waste factory pulp and paper (waste sulfite liquor) * gases hydrocarbon source of these materials on producing countries alcohol vary depending on the number of ingredients that can be obtained in countries that, for example: German: basic ingredients potato French: basic ingredients sugar beet Sweden: basic ingredients sulfate pulp Indonesia: basic ingredients molasses fourth. Raw materials 1. substance sakharin generally as media for the production of alcohol commercially in the industry fermentation of alcohol. In Indonesia used drops (molasses), which bisadidapatkan after sakharosanya crystallized and disentrifuse terms of cane sugar. The process of evaporation and pengkristslsn this usually done three times until tetestidak again economical to obtained. Remains drops / liquid is referred to as "black strap mollase" which is a complex mixture containing sakharosa, sugar invert, salts and materials non sugar. Besides sakharosa, glucose and fructose can fermented, molasses also contains substances reducing can not in the fermentation. These materials, among others, caramel YAG occurs because warming sugar, melanoidin containing nitrogen and there are also hydroxy methyl furfural, formic acid and others. Materials can not fermented this can be reached 17% in black strap mollase, and 5% in the high test mollase. Drops (molasses) acidic, have a pH 5,5-6,5 caused by the presence of organic acids free. Quality molasses generated from a sugar industry influenced by the way cleaning niranya. If not perfect, then dirt there are a lot in molasses. Color molasses in general reddish Brown. This is among others, pigments melanoidin, decoration thermal and chemical of the components in addition to sugar. 2. microbial frementasi in the process of fermentation alcohol use yeast. Yeast this can change the glucose be alcohol and co2. Yeast is microorganisms one-celled, not berklorofil and including class eumycetes. Of this group known several types, among others saccharomyces anamensis, schizosacharomyces pompe and saccharomyces cerevisiae. Each have the ability to produce alcohol different. Terms of the necessary in choosing yeast to fermentation are: · fast breed · stage of the high alcohol · resistant to high temperature · have the nature of stable · fast held adaptation of the media fermented to obtain the type of yeast have properties such as above, should be done experiments in the laboratory carefully. In general yeast used to make alcohol is the type of saccharomyces cerevisiae, which has the growth of the perfect at a temperature of ± 30oc and pH 4,8. Yeast according activities during the fermentation divided into two parts, namely: * top yeast (yeast above) yeast active on the surface of the media, which produce ethanol and co2 immediately. This type of usually found in the industry alcohol and wine. * Bottom yeast (yeast below) yeast active on the bottom. Usually industry producer beer that use yeast below are produces ethanol a little and takes a long time to perfection fermentation. In the condition of normal, yeast on inclined to berflokulasi and split from solution, when the fermentation running is perfect. Strain yeast varying the different in the ability berflokulasi. Factors that affect the life of yeast: * nutrients in activities yeast require the addition of nutrients for growth and breeding, for example: * elements C.: there's in carbohydrate * elements N: to the addition of fertilizer containing nitrogen. ZA, urea, ammonia, peptone and so on. * The element of P: to penmbahan fertilizer phosfat of NPK, TSP, DSP. * The Minerals * vitamins * acid (pH) for the fermentation alcohol, yeast require media atmosphere acid, that is between pH 4,8-5,0. Setting pH is done by the addition of sulfuric acid if substrate alkalis or sodium bicarbonate if substrate acid. * The temperature of the temperature of the Optimum for growth and breeding is 28-30oc. At the time of fermentation happen rise heat because of his reaction eksoterm. To prevent the temperature of fermentation not up, it is necessary cooling to be maintained remain 28-30oc. * The air fermentation alcohol takes place anaerobic (without air), However the air is required in the process of nursery before fermentation, for breeding yeast cells. V. the process of ethanol production basically there are 2 kinds of how the manufacture of ethanol, namely: · the synthesis · the fermentation the synthesis, conducted by using the reaction Elementary (hydration catalytic ethane), to change the raw materials into ethanol. As for the fermentation, done with the help of activity microorganisms. In this paper, discussion will be stressed in the process of ethanol production by way of fermentation. Fermentation bioethanol fermentation process ethanol can be done by using Baha-given material. For example material sugar like drops (molasses), and also the materials containing starch like Rice, corn, cassava, gandung and others. Fermentation process with different materials will certainly need the a rather different. The following is an explanation of the process of ethanol production with material molasses and material containing starch. The production process bioethanol of drops (molasses) 1. processing drops processing drops are important in the manufacture of alcohol.pengolahan is intended to get conditions yangoptimumkan to the growth of yeast and for the next. That need to be adjusted in processing this is a pH, the concentration of sugar and the use of nutrisi.tetes Yan faced of sugar factory usually still too package (850 Brix), therefore need to be held dilution first to mendapatkankadar sugar Optimum (120 Brix for nursery and 240 Brix padafermentasi) .pengaturan pH regulated by the addition of acid h2so4 to the achieved pH 4 - 5.meskipun drops contain enough substances nitrogen source However sepertiammonium sulfate or ammonium phosphate 2. stage weighing drops on weighing drops this used kind of scales quickly with a capacity of weigh particular, equipped with a tool opening and closing the form of valve waste that manually operated. And also the panel on-off pump drops are subject to automatically. How it works with considering drops pumped from warehouse storage drops for each day. 3. the stage of mixing drops. Stages of mixing drops using the tank mixing drops with a capacity of certain equipped radiant steam hot water (steam), which serves as a mixer and heating drops. How it works that pertamatama hot water temperature 70o C. put in the tank mixing drops (mixing tank), followed by the drops have weighed. After that circulated by using the pump up drops and water mixed with good. Mixing considered to be completed by the indication density reach 90 ° Brix and dipanskan with steam hot water (steam) to the temperature reaches 90 ° C. purpose given hot water is to speed up the dissolution, while the heating with steam hot water (steam) is to sterilization solution drops. After all mixed with good added sulfuric acid (h2so4) technical with density 96,5% to pH achieve 4,5 - 5. the provision of sulfuric acid (h2so4) aims to precipitate garamgaram Minerals in drops and to break down in-sakarida (sucrose) in the drops into monosaccharide the form of compounds D-glucose and D-fructose. 4. the stage of the deposition on the stage of the deposition of using the tank that comes with a pipe decanter. At this stage solution drops with density 40o Brix of the tank mixing accommodated in the tank and deposited for 5 hours to precipitate dirt-dirt drops (sludge), especially the deposition of Salt. Deposition aims to reduce the crust happened to mash column (column distillation first). After 5 hours, liquid drops pumped to tank fermentor through decanter and heat exchanger (he). Heat exchanger serves to lower temperatures up 30oc as terms of operation fermentation. While the fluid the rest of the form of sediment dirt-dirt and some of the liquid drops pumped to tank washers sediment dirt drops (the tank sludge). 5. stage separator tank washers sediment dirt drops. The rest of the liquid drops as much as ± 5% volume of the tank settling drops in the form of sediment dirt-dirt pumped out of the tank settling through the pipe decanter to fit in the tank sludge to achieve the volume of a particular. Then fluid drops precipitated up to a certain time for the next pumped back to tank mixing. Purpose laundering dirt drops this is for the efficiency of raw materials such drops to raw materials can be used as much as possible without having to throw some of the remaining. 6. the stage of fermentation fermentation process is divided into several stages, that stage breeding yeast and fermentation. Stages of breeding yeast this stage using the tank prefermentor equipped pipe flow of air and pipe flow cooling water on the outside of the Wall tank. This stage aims to breed yeast types of saccharomyces cereviseae by using the media drops. For the manufacture of solution yeast, at first started by entering process water temperature 15o C. and drops 40o Brix of the tank settling drops into the tank seeding and mix it up to achieve the thickness of about 12 - 13o Brix accompanied air flow of the blower with dual function is to accelerate tercampurnya drops with water and also for consumption oxygen demand for yeast saccharomyces cereviseae that takes place in the atmosphere aerobic. It also keep the temperature of the tank constant on 30o C. with the flow of water in the outer Wall tank. If not maintained, then yeast was bred will be disturbed survive and then going to die. Then enter yeast breads (gist), which have dissolved with enough water. For nutritional, put urea, diammonium phospat, and ammonia. PHP also added to this solution in order to maintain pH to remain constant ie 4.5 - 5. of the results of this mixture obtained culture yeast. In the tank is pre-fermentor there are some of the reaction: the reaction hydrolysis, the reaction of decomposition of urea and reaction growth yeast. Assumptions on the reaction hydrolysis is conversion happens 95%. Equation hydrolysis as follows: c12h22o11 + h2o 2c6h12o6 equation in 95% conversion process the decomposition of urea are: (nh2) 2co + h2o 2nh3 + h2o equation for the growth of yeast are: c6h12o6 + 3.198o2 + 0.316nh3 1.929ch1.703n0.171o0.459 + 4.098co2 + 4.813h2o (δhr 298 = -855.7055 KCAL / kg) (Atkinson, terms of 132) stage ferementasi this stage using the tank fermentor with equipped pipe flow of air and pipe flow cooling water from the River water to keep the temperature of fermentation on 30-32o C. fermentation aims to get alcohol with levels 8,5 - 9% or more. First started with sterilization tank fermentor yamg empty with steam hot water (steam) to temperature 121o C. and let the temperature in the tank down until 30o C. after that enter process water temperature 30o C., solution drops 40o Brix, fermentation process is run aerobic. The next culture yeast that has been bred in the tank is pre-fermentor pumped into the tank fermentor. After that, drops 40o Brix pumped into the tank and the last for 36 hours. For pH this solution guarded around 4,5 - 5. then enter yeast breads have dissolved with enough water and yeast cream. For nutritional, put urea, ammonium, and diammonium phospat. While the Turkey Red oil added as anti foam to prevent the formation of foam for the case. This is done for 15 minutes after the preparation of the media in the tank fermentor complete. Then put in 2 tank fermentor at the time adapted to the early hours of fermentation. Stage fermented this takes place for 24 hours to alcohol content achieve 8,5 - 9% and viscosity 6,5 - 7o Brix. After the alcohol content of 8,5 - 9% are met, a solution of the results of fermentation pumped to the separator to separated between the results of fermentation (liquid mash) with the yeast (yeast cream). Separator using the tools Rotary vacuum filter which is a tool to the principles of vacuum so yeast (yeast cream) and fluid results fermentation (liquid mash), which memilliki difference density can be separated. Yeast obtained still in high concentrations. From the results of fermentation not all separated raginya, only about 80-90% only. The rest of 10-20% not taken raginya because it contains kotorankotoran the rest of the form of sediment mineral salts. The results of fermentation that has been separated this directly into the tank mash (mash tank). And then didestilasi to be alcohol prima (fine alcohol) with the levels of reach 96,5%. On the stage of fermentation this occur reaction hydrolysis, where sucrose changed to glucose. Equation hydrolysis: the c12h22o11 + h2o 2c6h12o6 while the reaction main is reaction fermentation, where the glucose changed to ethanol and water. Equation reaction is: c6h12o6 2 c2h5oh + 2co2 on the main fermenter addition formed ethanol, would also formed side products. The results of the side in weight percent (% sugar) is as follows: acetic acid = 0,65% fusel oil = 0,85% asetaldehid = 0,05% (prescot it 128) adverse reactions that occur in the main fermenter: the c6h12o6 c3h8o3 + ch3cho + 2 co2 c6h12o6 + h2o 2 c3h8o3 + ch3cooh + c2h5oh + 2co2 (δhr 298 = -324.3860 KCAL / kg) components on fusel oil include: propanol = 12,5% isobutyl alcohol = 15% amyl alcohol = 30% isoamyl alcohol = 32,5% ethanol = 10% (paturau it 241) 7. stage distillation products fermentation alcoholic low, called the beer (beer) and therefore need in raise concentration by distillation bertingkat.beer containing 8 - 10% alkohol.maksud and the distillation is to separate the ethanol from campuranetanol water. To a solution that consists of components berbedanyata temperature boiling, distillation is the easiest way dioperasikandan is also the ways separation in thermal is efisien.pada atmospheric pressure, boiling water at 1000c and ethanol boiling padasekitar 770c. the difference in boiling point here is that allows pemisahancampuran ethanol air.prinsip: if the solution is a mixture of ethanol water heated, it will be more molecules ethanol evaporate of on the water. If the vapors this cooled (dikondensasi), then the concentration of ethanol in fluid condensed ituakan higher than in the solution of the original. If the condensate this heated again and then condensed, the concentration of ethanol will be higher. This process can be repeated continue, through most of ethanol concentrated in a phase. But it is no limit. The solution of 96% ethanol, obtained a mixture with boiling point the same (azeotrop). In these circumstances, if a solution of 95-96% alcohol is heated, the ratio of the water molecules and ethanol in the condensate will teap constant same. If the content etanolnya was 95% done dehydration or removal of the water. To remove the water can use calcium oxide or zeolite synthetic. Add calcium oxide in ethanol. Let overnight. After that distilled again until the levels of the water is approximately 99.5%. Fermentation ethanol from material containing starch the process of ethanol production of agricultural products containing starch (such as corn, wheat, and others) almost the same as the process of ethanol production with a basic materials molasses. However, in the process of fermentation this time, in the early stages will need additional process that is not performed on the fermentation molasses. Stages of the stage of his are as follows: 1. process gelatinasi in the process of gelatinasi, raw materials cassava, sweet potato, or corn destroyed dandicampur water so be pulp, which is expected to contain the starch 27-30 persen.kemudian porridge starch the cooked or heated for 2 hours so that shaped gel. Process gelatinasi can be done with 2 way, namely: porridge starch heated up 130oc for 30 minutes, then cooled sampaimencapai temperature 95oc estimated takes ¼ hours. Temperature 95oc such is maintained for about 11/4 hours, so that the total time required achieve 2 hours. The slurry starch plus enzyme termamyl heated directly to achieve the temperature 130oc for 2 hours. Gelatinasi first way, that is how warming gradually have the advantage, that is at a temperature of 950c activity termamyl is the most highest, resulting in yeast or yeast fast active. Heating with high temperature (1300c) on the way the first is intended to break down the granules starch, so much easier contact water enzyme. Treatment at high temperatures that can also serves to sterilization material, so the material is not easy contaminated. Gelatinasi second way, that is how to direct heating (gelatinasi with enzymetermamyl) on the temperature 130oc produce less well, karenamengurangi activity yeast. It is caused gelatinasi with enzyme in suhu130oc will be formed Tri-phenyl-furane who have the nature of toxic to yeast.gelatinasi at high temperatures also will affect the decline aktifitastermamyl, because the activity termamyl will be decreased after passing temperature 95oc (wasito, 1981). 2. the saccharifikasi stage sakarifikasi is a step-solving sugar complex be sugar sederhanayang performed on a tube in a series of equipment for the production of bioethanol.saccharifikasi involves process as follows: • cooling porridge until the Optimum temperature enzyme sakarifikasi work • setting pH Optimum enzyme • the addition of enzyme (glukoamilase) exactly • maintain pH and the temperature range 50 Elementary 600c, until the saccharifikasi completed (done by testing simple sugars generated). 3. fermentation fermentation process will run a few hours after all material put in fermentor. If you use fermentor that translucent Padang (from glass for example), then will seem bubbles air into small pieces from within fermentor. Bubbles air this is a co2 generated during the fermentation process. Sometimes sound roar during the fermentation process this. During the fermentation process is try to temperature not exceed 36oc and pH his maintained 4.5 - 5. the fermentation process is running more or less for 66 hours or approximately 2.5 days. One of a sign that fermentation done is look no further the bubbles the air. Levels of ethanol in liquid fermentation approximately 7% - 10%. 4. distillation and dehydration after fermentation process is complete, enter the fluid fermentation into the evaporator or boiler. Preheat evaporator and the temperature is maintained between 79 - 81oc. At this temperature ethanol was evaporated, but no water evaporated. Steam ethanol streamed to distilator. Bioethanol will be out of the pipe spending distilator. Distillation first, usually levels of ethanol is still under 95%. If the content of ethanol is still under 95%, distillation need to be repeated (reflux) to levels etanolnya 95%. If the content etanolnya was 95% done dehydration or removal of the water. To remove the water can use calcium oxide or zeolite synthetic. Add calcium oxide in ethanol. Let overnight. After that distilled again until the levels of the water is approximately 99.5% image equipment.

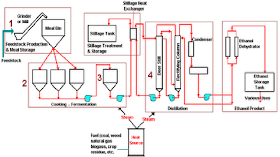
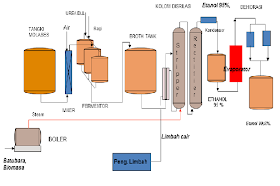
Vi. The usefulness bioethanol usefulness of ethanol / bioethanol (alcohol) based on the literature is as follows: according to Fessenden (1992) the usefulness of ethanol are: - used in the liquor. - as solvent and reagensia in the laboratory and the industry. - as fuel. Ethanol have calorific value (verse) of 12.800 BTU / lb. Whereas if mixed with gasoline where the percentage of 10% ethanol and 90% of gasoline will result in the product with trade name gashol generated heat (verse) of 112.000 BTU / gallon. According to Austin (1984) the usefulness of ethanol are: - as material chemical industry. - as material beauty and medicine. - as solvent and for the synthesis of chemical other. - as raw materials (raw material) to make the hundreds of chemical others, such as asetaldehid, ethyl acetate, acetic acid, etilene dibromida, glycol, ethyl chloride, and all of ethyl Ester. Under uhlig (1998) the usefulness of ethanol are: - as solvent in the manufacture of paint and materials komestik. - diperdayakan in the domestic trade as fuel. VII. Waste of the industry bioethanol according to hammer and Bastian (1989), wetlands are habitat transition between the land of land and water, so it is not a habitat land or habitat water. Ecosystem wetlands have a natural ability to eliminate the different types of waste on some level of efficiency (Nichols, 1983). The ability is mainly due to the vegetation role as a processing waste. Around the waters of land to be part of wetlands also serves as a storage and catcher carbon. More fantastic again, wetlands is also a buffer impacts of anomalies weather and climate. Thus, the potential of wetlands in Indonesia as warehouse carbon very large. Respond to the event the death of thousands of fish along 70km from mojokerto up Surabaya, events that occur due to pollution caused luberan waste University Arts, chemical archipelago. University Arts, chemical archipelago (akn) village Wates tenant mojokerto, is the industry producer of ethanol including the largest contributor organic pollution high times Surabaya. Can imagine to produce one liter ethanol generated waste 15 liters of molasses Brown, classified as a waste most corrosive bod (bio oxygen demand) and Cod (chemical oxygen demand) high, pH 3.5, high temperatures up to achieve 100oc can pollute the ground water. Molasses is the rest of drops of molasses that has been processed to produce sugar. Molasses containing 45% sucrose can fermented be alcohol. 1 kg sucrose theoretically will result in 0.644 liter absolute alcohol (anhydride) almost 100% pure. Mathematically with 88% efficiency fermentation 98% distillation will be generated 0.555 liters of alcohol. Bod (biochemical oxygen demand) is a number of dissolved oxygen required by bacteria pengurai to describe contaminants organic in water. The value of quality standard bod for drinking water should be the same or less than 2 mg / L. Cod (chemimal oxygen demand) is a number of dissolved oxygen required to untangle contaminants organic in water using oxidizing chemistry. The value of quality standard Cod for drinking water should be the same or less than 10 mg / ldi. Waste University Arts, chemical archipelago signing keperairan times Surabaya originally can Act as food items described by the microbial, but the decomposition of organic matter is in need of dissolved oxygen in water greater than the number of oxygen resulting from the process of photosynthesis. So the impact bad for aquatic organisms. In addition organic waste University ank cause four changes destabilize the ecosystem freshwater, namely: first. Organic waste containing suspended solids blocking penetration sunlight into the water body so that inhibit the process of photosynthesis. Second, the deposition of organic materials that settles will change the texture of substrate and lead to habitat that is not appropriate for Biota endemic in waters. Third, the formation of ammonia who have the toxicity of high and cause interference great for aquatic organisms and smell. Fourth, contaminants organic consists of compounds protein, carbs, fat and nucleic acid will increase the high concentration of bacteria and microorganisms pathogens. Hey coli is bacteria common in the bodies of water from the stool human or animal bloody hot as well as water has been contaminated by organic waste. This increase will bring the impact of pathogenic where bacteria and viruses contained in considerable amounts and harm health. Several types of bacteria water cause disease cholera, typhoid fever, dysentery basiler, and gastroenteritis. Virus there is also in the water termasul virus cause poliomyelitis, hepatitis infective. Animals parasites in water, among others roundworms ascaris and tapeworms cattle and pigs. All types of these organisms contained in stool contained in the sewer domestic and Ranch. Besides dilution by water, sedimentation to basic waters and decomposition of the Sun is also an important factor in the decomposition of organic compounds in waters. Decomposition by microbial will reduce the oxygen content dissolved in water, so the oxygen content that is not able to support the life aquatic organisms such as fish and other organisms. For that there is an effort offered to overcome this problem is the use of plants arise in waters for processing waste because these plants assimilate inorganic and organic compounds from waste. Plants with growth rate high and the Crown great can save a variety of nutrient Minerals. In the media gravel, plant growth arise can reduce the concentration of nutrient Minerals (laksham, 1979; Finlayson and Chick, 1983; Bowmer, 1987). Rizoma and root phragmites Australis scirpus SPP. serves as the filtration and settling compounds hydrocarbons and toxic heavy metals. The level of the concentration of heavy metals in the network plants are as follows: root> rizoma> leaves (shutes @ Inter alia., 1993). Plants floats such as water hyacinth can also get rid of nutrients and heavy metals in the number of significant (Reddy and debusk, 1985). VIII. Waste handling · condense waste evaporator. Then mengabutkan waste of concentrated in the furnace burning a temperature of 800 ° C. so organic materials in waste burned away. Abu combustion products it turned out to contain the potassium so processed to be the fertilizer · using waste bioethanol as raw materials fertilizer. Waste ethanol that often called vinase or distilet have the characteristics of a typical. Waste this can be used and processed into liquid organic fertilizer (POC). POC have sale price high enough so that can add value for ethanol industry. Vinase processed such a way that a product POC that can be nourish the plant. Application POC this can be used for all kind of plants, all commodities, and all the climate or place. Utilization POC can reduce or mensubtitusi the use of chemical fertilizers. POC of industrial waste ethanol this classified organic fertilizer, so the relatively more environmentally friendly. In the National scale pepanfaatan POC this can reduce the consumption of chemical fertilizers and mengemat state budget. If seen from the point of the industry, processing this can give income in addition, the industry. Waste treatment ethanol be POC quite simple and not too complicated. POC can be made with the cost of a fairly cheap and does not require equipment complicated. However, the manufacturing process requires accuracy and cautious. POC of vinases it could be combined with fertilizer other has outstanding on the market, such as fertilizer biological, or POC laiinya.poc made must dibuktukan before used in broad scale.
I Think You Are Chemist Student