The quest for elixir of life. Episode 5 - The tik-tok of telomeres
Ah! looks like I haven't posted here for months. We recently had a baby girl and she has kept me really busy and sleep-deprived. Though I kept writing but at a much slower pace. Anyhow, let's get to the topic now, I will talk about myself (or maybe not) in some other post. Now, since it has been quite some time I talked about ageing (probably it makes me feel even older now), I will restart with some basics and recap of what I have discussed earlier.

Image - profile of time, 1984 by Salvador Dali | Public Domain
What is ageing?
Ageing is the deterioration of life processes with time. It occurs because cells in our body age. More and more damaged and senescent cells accumulate in our body with time. The damage is multifaceted. To begin with, there is DNA damage that occurs in the cells with age. The pathways that repair DNA damage also slows down with age. Then, the mitochondria in the cells also get faces the consequence of ageing. As we age more damaged mitochondria accumulate in our cells. Mitochondrial biogenesis, the fission and fusion of mitochondria, in short, its turnover is compromised. These damaged mitochondria then produce free radicals known as reactive oxygen species or ROS. While on one hand ROS also works in cellular signalling it is also been accused of accelerating the damage to other cell components - DNA, proteins and lipids.
Now, the damaged cells have two ways to go. They can either die via apoptosis or hang in there with all that damage. When the senescence pathways such as p16ink and NfKb are turned on, chances are these senescent damaged cells will hang in there. The ROS, the NfKb and the inflammasome pathway, in these damaged cells then would secrete inflammatory cytokines and recruit immune cells (see more on infllammageing). The immune cells are also recruited by damage-associated molecular patterns and DNA secreted by dying cells. In any case, when the immune cells arrive they can clear up the damaged cells (though usually not the ones that have NfKb activated).
But, immune cells also age themselves. Over time they lose their ability to migrate and clear up these damaged cells. They rather start inducing further senescence in the nearby cells. This turns into a vicious cycle of more damaged cells, activating and recruiting more senile immune cells, and these immune cells cause an increase in the number of senescent cells. This leads to chronic low-grade systemic inflammation in the body. This chronic inflammation is known to be a cause (or at least it contributes) to many age-associated chronic diseases - such as obesity, diabetes, depression, anxiety, hypertension, myocardial infarction (heart attack), and many other neurological, fibrotic and autoimmune disorders.
The Tik-Tok within cells
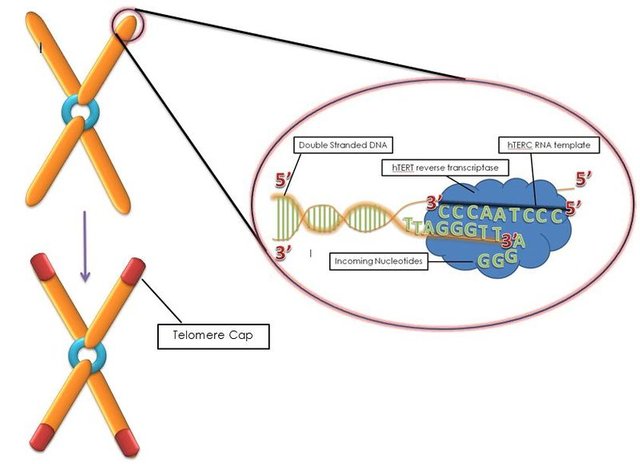
Image by developmentalbiology | CC BY-SA 3.0
Now the thing is that even if we found a way to fix the chronic inflammation, the mitochondrial dysfunction and the spontaneous DNA damage, there is another elephant in the cell. The elephant is known as the telomeres.
Unlike the circular chromosome of bacteria, eukaryotic chromosomes are linear. So while bacterial chromosomes are closed our chromosomes are in an open conformation. This makes our chromosomes prone to fraying and fusion with other chromosomes. Think of it as a book cover. Without the cover, the pages are more prone to damage. The solution - have a special sequence at the end of the DNA which can be capped by specific proteins and hence avoid the damage. Such sequence repeats at the end of the DNA are called telomeres - which means end (telos) parts (meres) in Greek.
Anyhow, every time the DNA has to replicate before the cell divides, it can't copy the end of chromosomes. This is known as end replication problem (ERP). On one hand, telomeres ensure that the end replication problem doesn't affect the genes on the chromosomes. On the other hand, telomeres themselves suffer the consequences of ERP. Hence, with every cell division, the telomeres shorten. The short telomeres would then lead to turning on a DNA damage response, cell cycle arrest, and senescence (if the cell doesn't die via apoptosis). The senescent cells would them lead to inflammation as discussed before.
Cellular clocks and our time in the mortal world.
Man by Jo-B and Mouse by Dridibenacher | Pixabay
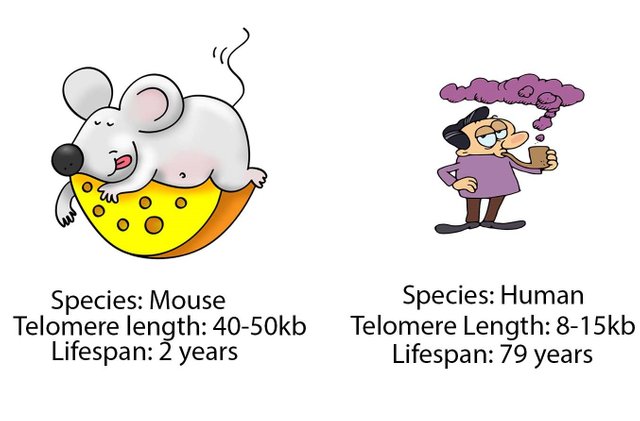
If the telomeres hypothesis of ageing is to be considered then it would imply that organisms who have longer telomeres should age slower and live longer. However, this doesn't hold true and is a major criticism of the hypothesis. For instance, mice have 10 times longer telomeres than humans. However, they age rapidly and have quite a short lifespan - approx 30 times shorter than humans (Rodrigo et al., 2013).
An internal matter of species?
Telomere length
Nevertheless, it has been shown that in same species, such as mice, individuals made with embryonic stem cells with increased telomere length, age slowly and live longer (Miguel et al., 2019). This is quite interesting. Not only because these mice show slower ageing, but also because this paper shows that these mice with longer telomeres are leaner. They have less fat accumulation though there was no change in lean mass. The metabolic parameters are better in mice with longer telomeres, indicating the role of telomeres in the metabolic arm of ageing. Though, it is to be noted that even though the length of the telomeres was doubled in these mice, but it only led to 8.4% increase in maximum lifespan (and they still did not outlive humans).
Well, while one reason that telomere correlation doesn't hold true between species but does within species might be the rate of degradation of telomeres is different in different species. For instance, it has been shown that telomeres degrade 100 times faster in mice than in humans (Vera et al., 2012). So if we were to slow down the telomere degradation in mice close to humans would they outlive humans?
The watchmaker - Telomerase
We know that in stem cells there is an enzyme called telomerase which can synthesize telomeres and hence maintain their size, and slow down their degradation. Mutations in this enzyme in humans and mice have been shown to accelerate age-associated pathology. (Herrera et al., 1999, Garcia et al., 2007).
Now obviously, having telomerase being expressed in stem cells is not really slowing down telomeres degradation enough in mice. Which might be because in the non-stem cells short telomers can contribute to ageing via cellular senescence or even in stem cells the amount of telomerase is not sufficient to keep the length of the telomeres constant. So what if we externally increase the amount of telomerase in all cells? That should slow down the degradation. Jesus et al., 2012 (no resurrection joke intended) showed that overexpressing telomerase in adult mice via gene therapy increases the life expectancy by 24% and max life span by 13%; apart from slowing down the overall age-associated tissue degradation. And, overexpressing it in old mice increases maximum life span by 20%. However, what if mice were born with excess telomerase, to begin with?
Well, there is one issue with that - while telomerase overexpression has been shown to increase lifespan, it also contributes to a higher incidence of cancer (González-Suárez et al., 2005). Nevertheless, overexpression of telomerase in cancer-resistant mice can increase the life expectancy by about 26% compared to just the cancer-resistant mice (Tomas-Loba eta al., 2008).
Now, neither making mice with excessive long telomeres nor overexpressing telomerase can explain the overall lifespan differences between species. However, we cannot deny the fact that telomeres do contribute to ageing as long as we consider individuals of the same species. This, definitely means that the life span of any species is dependent on factors other than just telomeres. This can be their metabolism, the DNA repair mechanism, their immune system etc. It will be interesting to see how these different factors involved in ageing synergistically contribute to overall species lifespan? Do they add up linearly or in some non-linear fashion?
It is to be noted that, Miguel et al., 2019 showed that telomere shortening is also tissue-dependent. That the rate of telomere degradation is different in different tissues. In their study, the major degradation of telomere occurred in adipose tissue. And since adipose tissue is an important part of metabolic regulation, it is important to consider metabolic aspects of species being compared.
Adjusting the clock with calories
In my previous blogs, I have pointed out that caloric restricting until now is the best-known technique for slowing down ageing. We have talked about how caloric restriction improves ageing of the immune system, age-related chronic inflammation, mitochondrial function, and even DNA damage and repair. Hence, it becomes important to ask if caloric restriction also have any effect on telomeres degradation and revival. Vera et al., 2013, showed that on one hand, calorie-restricted mice showed slower degradation of telomeres. So caloric restriction does have an effect on telomere related ageing as well. Nevertheless, on the other hand, Vera et al., also showed that overexpression of telomerase combined with caloric restriction further increased lifespan of these mice. This again indicates that there is more than just telomeres that contribute to ageing.
Closing remarks
In a nutshell, what we learnt today is that while on one hand there is a cellular clock that does impact ageing. However, it is neither independent of other mechanisms nor is the only reason behind ageing. It remains to be seen that different aspects and pathways of ageing that we discussed, can explain the differences of lifespan between species. Do let me know if you have come across any such studies which try to understand the bigger picture rather than parts of the picture.
About steemstem
But, before I go I would like to mention about the steemstem platform. Well, if you love reading and writing interesting science articles @steemstem is a community on steem that support authors and content creators in the STEM field. If you wish to support steemstem do see the links below.
You can vote for steemstem witness here -Quick link for voting for the SteemSTEM Witness(@stem.witness)
Quick delegation links for @steemstem
50SP | 100SP | 500SP | 1000SP | 5000SP | 10000SP
Delegating to @steemstem gives an ROI of 65% of the curation rewards.
Also, if you have any questions regarding steemstem, do join the steemstem discord server.
You can DM me on discord, I have the same handle - @scienceblocks. Also if you are not a steem user, and reading this blog inspired you to start your science blog, find me on discord and let me know about you. I can try and help you navigate your way through steem.

References and further reading
The quest for elixir of life - understanding caloric restriction (episode 2 - mitochondria)
he quest for elixir of life - Episode 3 - Inflammageing
The quest for the elixir of life - Episode 4 - Tackling immunosenescence
Wnyford-Thomas and Kipling, 1997. The end-replication problem
Miguel et al., 2019. Mice with hyper-long telomeres show less metabolic aging and longer lifespans
Tomas-Loba et al., 2008. Telomerase Reverse Transcriptase Delays Aging in Cancer-Resistant Mice


Wonderful to see you back, and congratulations on the new family member. I wonder, as you nurture her, how you will be influenced by your research.
Your article is particularly interesting to those of us who have read your previous posts and what I see as almost complementary posts by @chappertron on mitochondria.
I think I understood almost all of this (that's a tribute to your writing!) One part of the discussion that comes through is that fat is implicated. Perhaps it's not caloric intake as much as the amount of fat in a body that affects lifespan. Excess calories are stored as fat, are they not? And is there a difference between the telomeres in so-called brown fat vs white fat?
Thank you for this most interesting discussion. Have fun with the baby. If you have fun, so will she :))
Regards,
AG
Thanks @agmoore2. As far her nurture and my reasearch is concerned I have learnt a lot about affects of stress during early childhood on rest of the life. And I keep telling anyone involved in taking care of her to be super careful at least during first 5 years of her life. Idk, maybe I am just a little paranoid.
Fat obviously worsens the situation. Excess fat is known to increase chronic low grade inflammation which would then accelerate ageing for sure. For the excess calorie been stored part is quite true, however, we also need to consider what makes up those calories. Not every calorie is the same and lead to equivalent storage of fat. I think I have mentioned this somewhere previously that we do know calories overall impact ageing but which calories are more important than others remains an open question.
If you look at the paper by Miguel et al., show significant decrease in short telomeres in all metabolically relevant tissue - liver, WAT and BAT. And decrease in telomere loss in these tissue seems to be significant in metabolic ageing. But yes you are correct, some needs to delineate impact of telomeres from metabolic context in these tissues.
Also, the whole calorie or fat impacting ageing is a complex nexus. While on one hand fat seems to accelerate ageing there is also an independent component of ability of cells to understand the nutritional state of body and respond accordingly.@valued-customer points out interesting pathways in this context in their comment. The low-energy state can push the cells towards maintenance mode. (But be careful when it comes to low vs starvation, starvation is detrimental).
Thank you for that comprehensive response. I think, until science offers more definitive answers, the path forward for all of us (nutritionally) is to follow a path of moderation.
As for your child...so lucky to have a parent with the goal of reducing stress. Most parents pressure their kids to do this or that, to achieve, to meet some imagined goal. But you understand the toll ambition demands on a child. I never pressured my children, but I was under great stress as a young mother learning her way. I understand so much more now. I think grandparents make better parents. Why does nature arrange things that way? :))
Have a great day, and enjoy your daughter.
Regards, AG
Superbly researched and conveyed.
Interestingly, I have just today read research that may impact your interest. It is notable that centenarians are far more likely to be smokers than not, which seems extremely counterintuitive to many unfamiliar with the nootropic and metabolic effects of nicotine.
This and more is published at sott.net, with copious cites available there.
Thanks!
@tipu curate
Upvoted 👌 (Mana: 25/30 - need recharge?)
Hi @scienceblocks!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation!
Your UA account score is currently 3.997 which ranks you at #4111 across all Steem accounts.
Your rank has dropped 4 places in the last three days (old rank 4107).
In our last Algorithmic Curation Round, consisting of 96 contributions, your post is ranked at #57.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.