Let's Learn Something Cool - Liquids That Levitate (The Leidenfrost Effect)
Hello steemians! Another week has just begun and another short educational post is here to give you an explanation on something we have all witnessed at least once in our kitchens but never really bothered as to why it happens.
I am sure you have seen the case when drops of water fall on the burning hot stove top but instead of evaporating the start doing the "sizzling dance". I remember as a kid I used to pour water over the hot burners after my mum had finished cooking and watched the droplets dance (yeah, I was a weirdo. I also like to watch clothes spinning in the washing machine, maybe I'm a bit autistic, who knows?).

(Image source: commons.wikimedia.org)
Now, let's get back to the hot stove tops. This happens for a very simple reason explained by the Leidenfrost effect (when I first read about it I thought it was going to talk about ice, but it's actually the total opposite). The effect got its name from the scientist that first observed it, doctor Johann Gottlob Leidenfrost, somewhere in the 18th century. Another name given to it is also "film boiling".
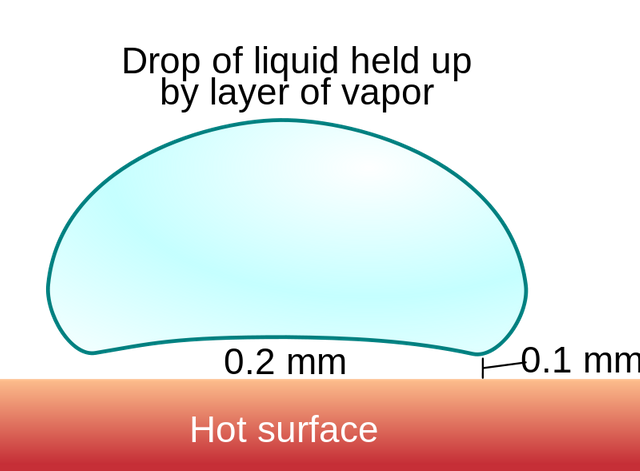
Put it simply, please.
When you pour water over a hot pan it starts getting heated up. When the temperature is below the boiling point, the water starts evaporating slowly. As the temperature in the pan reaches 100 °C, water starts boiling, and water droplets turn from liquid to gas. But what will happen if we keep providing heat and the temperature gets a lot higher?
Well, when a liquid falls on a very hot surface with a temperature extremely higher than the liquid's boiling point, then the surface of the droplet that touches the pan immediately turns to steam, building a "protective, insulating layer" where the rest of the water lies on. Since steam is a bad heat conductor, this means that the droplets life expectancy is elongated.
Calculating the temperature of the Leidenfrost effect is not always easy because lots of parameters are to be considered, such as the liquid's impurities and the surface's properties. But for water we can say that's about 193°C.
Safety Note: DO NOT TRY THIS AT HOME
For the most brave of you out there, there have been demonstrations of the effect where you can first dip your hand in water and then in a container of molten lead. If everything is done right, your hand will remain intact for a few moments, before the scorching lead starts roasting your flesh. The same happens in you dip your hand in liquid nitrogen. With a boiling point of - 195.8°C, your hand's temperature will be way hotter than that and an insulating "steam-glove" will protect you for a very little while.
(Image source: en.wikipedia.org)
Watch this video from the SciShow guys, I simply love them!
And if you want to satisfy your curiosity and see for yourselves, then follow this guy's instructions:
References
wikipedia.org_1
wikipedia.org_2
iflscience.com
comsol.com
physlink.com

Thank you for stopping by and reading this post! If you liked my work you can always visit my blog, @ruth-girl, and check out my previous posts like the bizarre natural phenomena series and lesson plans.

Finally, for those of us engaging with education, @steemiteducation is here to join all steemian educators in their common cause of making our job easier, more effective and more fun, because...

(Original image credits: Nick Youngson - nyphotographic.com)
Thank you for your time and as I like to say,
Steem on and keep smiling, people! :)

This post has caught the eye of @MuxxyBot and has been nominated by the curation team.
If chosen it will feature in a curation post by @MuxxyBot.
An image from your post may be featured.
Please reply to this comment if you accept or decline.
Muxxybot is a Curation account that features chosen posts, selected and voted on by the Curation team. If featured, your post will be shared on Muxxybot's post, which will be resteemed by @gmuxx. The author will then get added to Muxxybot's voting list for automatic votes on all future posts.
Thank you!! I accept of course! :)
Thank you!
good post, I suport
I folow you good ser please folow back
Great post @ruth-girl. thanks for sharing. Expect some more posts on science in near future.
Thank you dear!! Stay tuned for more ;)
This post has received a 1.33 % upvote from @buildawhale thanks to: @ruth-girl. Send at least 0.100 SBD to @buildawhale with a post link in the memo field to bid for a portiona of the next 100% upvote.
To support our curation initiative, please vote on my owner, @themarkymark, as a Steem Witness
I could watch water droplets hovering on a stove top like this for hours. Wasn't familiar with that Leidenfrost effect though. Glad I learned something new :-)
Nevertheless, even with this new found knowledge, I wouldn't want to try the experiments and dip my hand in anything with significantly different temperatures 😱
I'm happy that you found my post educational, @reinhard-schmid!
Well, I wouldn't be such a daredevil either! I love my hands...both of them! :P
Great post! I always forget the name of this effect.
Thank you!
Personally, it reminds me of a german dictionary called "Langenscheidt" :P
this post is so amazing, I'm so inspired to keep working hard on steem.
My wife constantly grumbles when I accidentally splash water on the stove, because stains remain on ceramic coating after that. But now, I'm gonna to tell her:
Hahaha!
Yes, don't worry love, it's just physics magic!
@ruth-girl will write a post on how to easy clean the stains with natural ingredients so I can play with the cooker all the time :P
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by ruth-girl from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.