Hafnium in control rods for nuclear reactors
A brief :
Nuclear science and technology have had, have and will have a great influence on society. As a researcher, I consider it an obligation to devote part of my time and efforts to share the knowledge that I have acquired and generate in this field. my intention is to make a serious and objective disclosure on issues related to nuclear science and technology.
Recently the use of hafnium as a neutron absorber material in nuclear reactors has been the subject of investigation, under which this material has nuclear properties in terms of neutron absorption and structural factors, which can extend the useful life of the control mechanisms of the nuclear reactors. In this post, I present some of the
most significant properties of hafnium as a nuclear material. They have presented also calculations made with the HELIOS code for fuel cells of uranium oxide and of mixed oxides of uranium and plutonium in controlled with conventional boron carbide bars and also with bars similar to which the absorbent material was replaced by metallic hafnium.
What is the hafnium?
Hafnium is a chemical element of the number 72 found in group 4 of the periodic table of the elements and is symbolized as Hf.
It is a transition metal, bright, silvery gray, chemically very similar to zirconium, found in the same minerals and compounds, and is difficult to separate. It is used in alloys with tungsten in filaments and electrodes. It is also used as s
a material for control rods of nuclear reactors due to its neutron absorption capacity.
1. Hafnium as a Absorber
One of the most desirable characteristics for a neutron absorber material is that it maintains its absorption efficiency during the entire life of the reactor, characteristics that hafnium seems to gather through numerous mechanical, chemical and long-term radiation tests.
Natural hafnium consists of six stable isotopes, each of which has an effective section of neutron absorption considerably high, so many Researchers have established that hafnium can be used for long periods of time under irradiation without significant loss of physical efficiency. Control mechanisms manufactured with hafnium and irradiated for 15 years until fluences of 3x1022 n / cm2, decreased their efficiency by approximately 6% to 7%. This is because, when a hafnium atom absorbs a neutron, a new isotope with high absorption efficiency. This decrease in efficiency comes in a slower way than in the Ag-In-Cd alloy control rods.
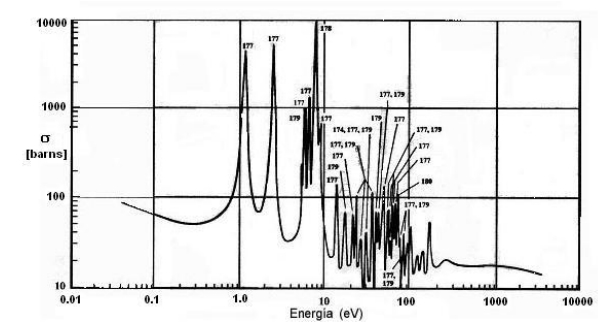
The structure of the resonances of the hafnium is very complex, it has seven peaks of resonance with high values of effective section located in a neutron energy range of 1.0 to 100 eV, because the capture by resonance is considerable,
The efficiency of the control mechanisms manufactured with hafnium does not depend on the power exchanges of the reactor.
2. Mechanism of positioning of a control bar
The control bars are grouped, for the best control, in banks so that all the bars of a bank are usually moved in unison, as a unit. The bars of a bank are symmetrically arranged in the core so as not to create radial distortion of flow during its movement.
The stop margin is defined as the instantaneous amount of reactivity by which the reactor would become subcritical from its present condition assuming that all banks are fully inserted except for the one with the highest antireactivity that is assumed to remain fully extracted. By means of the stop margin it is ensured that: a) the reactor can be made subcritical from all operating conditions, b) the reactivity transients are controllable within acceptable limits and c) the reactor will remain sufficiently subcritical to avoid accidental criticality in the condition stop.
For more information you can check the following content:
- https://en.wikipedia.org/wiki/Hafnium
- Meija, J.; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- EnvironmentalChemistry.com. "Hafnium Nuclides / Isotopes". Periodic Table of Elements. J.K. Barbalace. Retrieved 2008-09-10.