PART 1: EDIBLE FUNGI & THEIR ROLE IN EXERCISE-INDUCED IMMUNOSUPPRESSION
Edible fungi are accepted as functional foods. They have been extensively proven to provide therapeutic benefits beyond their nutritive value (1, 6). The scope of compounds and derivatives that can be extracted via fungi and their subsequent utility hold great promise both for the average consumer, amateur/professional athlete and for the nutrition-health relationship that practitioners foster with their clientele (1).
I consider mushrooms inherent functional foods for two main reasons:
Fungi, just like us, share highly conserved nutrient-sensitive pathways, such as the TOR pathway (Figure 1) (2).
Fungi produce β-glucans of differing molecular weight and solubilities, which possess antifungal and anti-tumour activities that stimulate innate immunity: the expression of pattern recognition receptors (PPRs) such as the Dectin-1 signalling pathway along with toll-like (TLRs) and mannose receptors. (Figures 2 & 3) (3, 4).
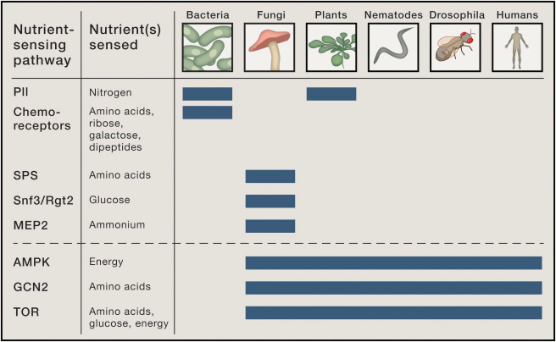
Figure 1. Nutrient-Sensing Pathways
Image source (2)
Intensive training schedules can functionally depress athletes’ immune systems if their nutrition is inadequate. This, coupled with other environmental stressors, can increase the risk of infection. Realistically, a combination of high-intensity and impaired recovery may be the ‘open window’ that can then further increase an athlete’s risk of infection (5).
Logically, tailoring an athlete’s nutritional intake not only aids in immune function, but recovery as well. However, methods based on heterogeneity, exercise protocols and other individualistic characteristics make tailored nutrition a complex array of multidisciplinary approaches and tests. How can one narrow down and tailor the vast and complex array of programmes? One example may be approaches that target these conserved and adaptive systems. A functional medicinal viewpoint may provide an approach to combat exercise-induced immunosuppression and assist in recovery. This can be achieved via nutritional strategies that incorporate edible and medicinal fungi, namely: Shiitake, Oyster varieties, Lions mane, Maitake, brown and white Shimeji, and others (6).

Figure 2. Active Compounds from Mushrooms (Martin Powell, 2013)
Image source cited in (10)
Naturally, just as we have a main structural constituent called keratin in our skin, plants have cellulose, and crustaceans and fungi have cellular walls composed of chitin. Chitin, through extraction methods (Figure 3), breaks down into further derivatives, including β-glucans. The water soluble 1→3 β-glucans, being the most bio-actively available via standard heat-treated procedures, enables us to utilise cooking methods as our most cost-effective, low-level extraction method. β-glucans are water-soluble, low molecular weight and easily extracted once chitin has been broken down. These derivatives can function as the nutritional elements driving weak innate immune signals through Dectin-1. Dectin-1 is a super family of receptors in humans, that senses β-glucans from plants and fungi (4).
However, chitinases are needed to breakdown chitin. These enzymes are present in mammalian stomach acid. Despite being a small enzymatic family, not all are active and rely on complex cellular signalling via host-defence and a working theoretic of interspecies communication (7). In summary, the way in which glutamic acid is substituted for glutamine, leucine, and iso-leucine influences chitinolytic activity, as glutamic acid forms the active site of mammalian chitinases (4).
Thus, some of the inherent innate immuno-stimulation and immuno-modulation of edible mushroom consumption can be attributed to the nutritional profile of edible mushrooms (Figure 2) (4). A full BCAA profile is encompassed in a protein content of 250g/kg of mushroom dry matter (6). Once you factor in the presence of mannitol, secondary metabolites, β-glucans and accompanying receptors such as Dectin-1, mannose and TLRs, you can start to unravel the complex interplay between the activation of innate immune cascades and how mTOR, the master regulator of protein synthesis and amino acid-sensing (Figure 1), functionally differentiates levels of pro- and anti-inflammatory cytokines produced by innate immunity on an ad hoc basis (1, 4, 6, 8, 9). The totality of the bio-active compounds in mushrooms, and how these compounds signal nutrient sensitive and signal tranduction pathways, is what makes fungi a natural functional food (1). However, our ability to access these compounds in Figure 2 is highly variable, depending on how mushrooms are prepared, the extraction process and the specific methodology behind the mushroom cultivation life cycle (Figure 3). This will be all discussed further in Part 2.
Figure 3
Image source cited in (10) (Martin Powell, 2013)
References:
- Reis, F., et al., 2017. Functional foods based on extracts or compounds derived from mushrooms. Elsevier, Trends in Food Science & Technology, Vol. 66, pp. 48-62. [online]. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0924224417300699.
- Chantranupong, L., Wolfson, R., L., and Sabatini, D., M., 2015. Nutrient-Sensing Mechanisms across Evolution. Cell, Volume 161, Issue 1, p67–83, 26. [online]. Available at:
https://www.cell.com/cell/fulltext/S0092-86741500242-1 - Lee., D., H., and Kim H., W., 2014. Innate Immunity Induced by Fungal β-Glucans via Dectin-1 Signaling Pathway. International Journal of Medicinal Mushrooms, Vol. 2014, pp. 1-16. [online]. Available at: http://www.dl.begellhouse.com/journals/708ae68d64b17c52,5607443c009a2341,22a4a957201ac47c.html.
- Batbayar, S., Lee, D. H., & Kim, H. W. (2012). Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomolecules & Therapeutics, 20(5), 433–445. [online]. Available at: http://doi.org/10.4062/biomolther.2012.20.5.433
- Wolfgang, G., Konrad M., and Pail, E., 2012. Exercise-Induced Immunodepression in Endurance Athletes and Nutritional Intervention with Carbohydrate, Protein and Fat—What Is Possible, What Is Not? Nutrients. 2012 Sep; 4(9): 1187–1212. [online]. Available at:< https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3475230/>.
- Valverde, M., et al., 2018. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int J Microbiol. 2015; 2015: 376387. [online]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4320875/.
- Konopka., J., 2012. N-Acetylglucosamine Functions in Cell Signaling. Scientifica, Volume 2012, Article ID 489208, 15 pages. [online]. Available at: https://www.hindawi.com/journals/scientifica/2012/489208/.
- Wolfson, R., L., and Sabatini, D., M., 2017. The Dawn of the Age of Amino Acid Sensors for the mTORC1 Pathway. Volume 26, Issue 2, p301–309. [online]. Available at:https://www.cell.com/cell-metabolism/fulltext/S1550-4131(17)30422-9.
- Brown, J., et al., 2011. TLR-signaling Networks. J Dent Res. 2011 Apr; 90(4): 417–427. [online]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075579/.
- Martin Powell., 2013. Medicinal Mushrooms The Essential Guide. CreativeCo Ltd, 35A Monument Business Park, Chalgrove, Oxfordshire. ISBN 978-0-9566898-1-8.
Welcome to Steemit! Your post is brilliant! However, there is a potential problem with the copyrights for the figures from Cell and other Journals.
Please visit steemSTEM channel on Discord, and find the detailed guidelines for steem STEM posts.
If you manage to change potentially conflicting images with the PD, CC0 or similar (from Arxiv or some Open Access, but please, check if they allow usage without restrictions), leave me the response.
Good luck! :)
Hi there Alex
Thank you for the feedback, I was on the Cell site however there is no link to request permission for the Cell article (2).
I have edited the potential issues, will you kindly confirm if there is still CC0 conflict?
Thanks again, much appreciated.