CATHODIC PROTECTION; CURBING THE MENACE OF CORROSION PART 1
When a piece of metal is exposed to the environment, certain changes begin to occur in the metal, and it gradually reduces in size and strength with the formation of certain deposits. Sometimes referred to as erosion, corrosion is the deterioration of a material as a result of its interaction or reaction with its environment.
Corrosion refers to a natural process which converts a refined metal to a more chemically-stable form, such as its oxide, hydroxide or sulfide. It is the gradual destruction of materials (usually metals) by chemical and/or electrochemical reaction with their environment.
It can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common to be used. When corrosion occurs in materials or structures, useful properties such as strength, appearance, and permeability to liquids and gases are degraded and thus reducing the quality of the material in question. The corrosion of metals is an electrochemical process. It is the physical interaction between a metal and its environment which results in changes of the metal’s properties and which may lead to functional impairment of the metal, the environment or the technical system of which they form a part.
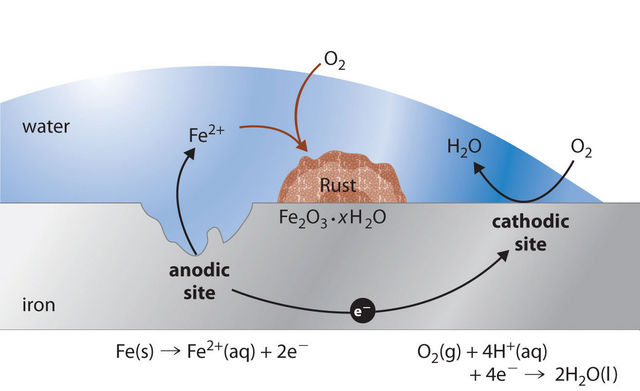
The most common product of corrosion is rust which is the reaction product of iron and steel corrosion.
In the industries that require the use of storage or transport facilities such as underground storage tanks, overhead storage, pipelines and the likes, the menace of corrosion is not a new thing and trust me, it is a serious matter that is always given considerable attention.
The effects of corrosion on the individual and collective lifestyles of humans cannot be overemphasized. Corrosion, if not properly managed, can lead to highly catastrophic occurrences and also might lead to high costs of production and maintenance of industrial structures.
The overall cost-effectiveness of corrosion in industries is usually on the high side. One of the most significant challenges facing our aging infrastructure today is materials loss and deterioration by electrochemical reactions that cause corrosion. Many government studies indicate that in the US alone, costs due to corrosion loss is more than $276 billion annually.
The severe consequences of the corrosion process have become a problem of worldwide significance. In addition to our everyday encounters with this form of degradation, corrosion causes plant shutdowns, waste of valuable resources, loss or contamination of product, reduction in efficiency, costly maintenance, and expensive overdesign; it also jeopardizes the s safety and inhibits technological progress. Corrosion also affects the appearance of structures; ta, e for instance, a motor vehicle, the presence of corrosion would reduce the riding comfort, the lifespan and also the resale value of the car. As much as aesthetics is an important factor considered in the design of structures and equipment, corrosion is one principal ‘enemy.’
When corrosion occurs, it tends to return the metal to its initial state.
Corrosion returns the metal to its combined state in chemical compounds that are similar or even identical to the minerals from which the metals were extracted. Thus, corrosion has been called extractive metallurgy in reverse. This corrosion occurs as a process in which current leaves a structure at the anode site, passes through an electrolyte, and re-enters the structure at the cathode site. Both the type of metal and the environmental conditions, particularly gasses that are in contact with the metal, determine the form and rate of deterioration.
Take for instance a simple electrochemical cell where the anode material loses electrons and is used up with time; the corrosion cell is also a simple replica with the environment (soil, water or moist atmosphere) being the electrolyte.
There are different forms in which corrosion can occur, these include; uniform corrosion, crevice corrosion, pitting corrosion, hydrogen damage e.t.c.
The driving force that causes metals to corrode is a natural consequence of their temporary existence in metallic form. To produce metals starting from naturally occurring minerals and ores, it is necessary to provide a certain amount of energy. It is therefore only natural that when these metals are exposed to their environments, they will revert to the original state in which they were found. Corrosion affects virtually every material or structure that is made of metal or has a metallic component and is exposed to the environment.
In the making of steel, when the iron is divorced from the associated oxygen in the blast furnace, a lot of energy is put into it in the form of heat. As long as it remains metallic, a piece of steel retains this energy bound up within it, always urging the metal to corrode back to the ore from which it was unwillingly derived.
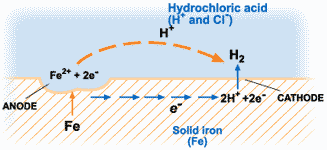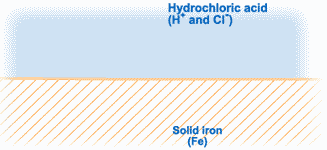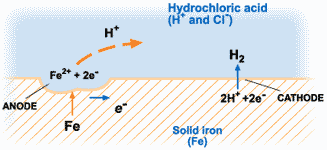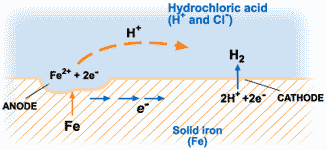
The corrosion of metals is an electrochemical process. That is, it is an electrical circuit where the exchange of electrons (electricity) is conducted by chemical reactions in part of the circuit. These chemical reactions occur at the surface of the metal exposed to the electrolyte. Oxidation reactions (corrosion) occur at the surface of the anode and reduction reactions occur at the surface of the cathode.
For corrosion to occur, the presence of oxygen, water, and the metal must be available. Corrosion will not happen if one of these elements is absent entirely. Corrosion involves both an anodic and cathodic reactions. The anodic reaction requires metal dissolution (corrosion), and the general equation for metals experiencing corrosion is given thus;
M →M^n+ + ne^-
Where M is the given metal and n is the number of electrons.
A common example is:
Fe → Fe2+ + 2e-
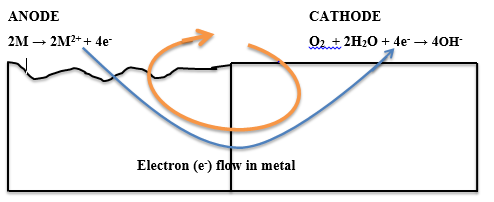
The electrochemical process of corrosion consists of four distinct parts: anode, cathode, electrolyte, and metallic path. These four components constitute what is called the “corrosion cell.” Electrochemical corrosion occurs only when all four parts of the corrosion cell are present.
Some factors which influence metal corrosion and the rate of corrosion are the; Type of metal; heat treatment and grain direction; presence of a dissimilar, less corrodible metal (galvanic corrosion); anode and cathode surface areas (in galvanic corrosion); temperature; presence of electrolytes (hard water, salt water, battery fluids, etc.); availability of oxygen; presence of different concentrations of the same electrolyte; presence of biological organisms; mechanical stress on the corroding metal; and time of exposure to a corrosive environment.
Some other factors include; ph of the electrolyte (soil or water body), the reactivity of the metal, the presence of electrolytes, impurities, temperature, e.t.c.
Well, I am sure the first thing that comes to the mind of an average human is ", RUST" but I can authoritatively say that there is more to the word than meets the eye.
A whole lot of bad things can be said about corrosion and its effects to human life and activities, but the good news is, there are practical methods which help to curb the menace of corrosion, one of which is the cathodic protection method, which fortunately is applicable in a large scale.
I am currently working on my undergraduate project on corrosion protection by an impressed current, and I will gladly share information about it in due time. In my next post of this series, I would be writing on the methods of combating corrosion.
Thank you for reading. A big thank you to @tormiwah for his tips and support
REFERENCES
CORROSION
WHAT IS CORROSION
TYPES OF COROSION
Effect of anode and size variations on the cathodic protection of mild steel in seawater and sulphuric acid
RESEARCH GATE -CORROSION
INTRODUCTION TO CATHODIC PROTECTION
All images are sourced in the post and stated to be in the public domain

Raphelle this is so awesome.. It indeed contains a lot of knowledge. If only you read this for corrosion engineering last semester.
Lol @adetola at least i am glad i read it now
Soo much knowledge here.
Good work.
Would love an upvote and a follow back.
Merry Christmas and God bless you.
Thank you
Mr. Corrosion