INTRODUCTION TO BASIC CHEMISTRY AND CHEMICAL CONCEPTS FOR BEGINNERS
Yes that strange dot-like stuff in the image above. Do you know? I guess not! me neither.
Some books claim that they are what we know as #Ions. hmm ions?
Yes ions!!
So what are this ions exactly?
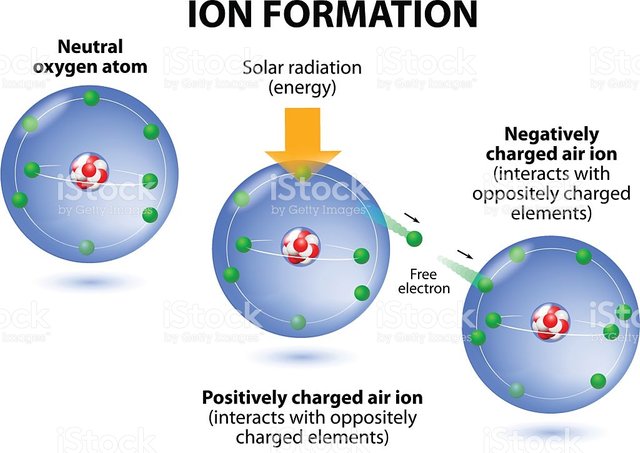
Scientist like Dalton claim that this so called ions, are tiny particles that are electrically charged; meaning they exhibit both positive and negative charges in their chemical states.
So are ions just particles that inhibit specific charges?
Well not really.
Ions aren't just particles that emit charges, they are so much more than that. In-fact did you know that ions make up most of all objects and structures you see around. You may have been taught in school that all living and non-ling things are made up of matter(a substance that has weight and occupies space); well matters are also made up of those particles as ions. And when coupled up closely together, they form different objects and substances.
Ions are so tiny that they cannot been seen with the naked eye, unless viewed using a complex and advanced microscope. That why, a lay man would never believe they exist.
Particles that make up ions are also called atoms and atoms are the smallest particle of an element which can take place in chemical reactions. That means even ions takes place in a chemical reaction and electrochemical reaction.
These electric charges produced by ions are:
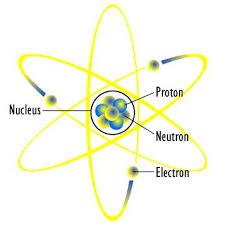
- positive charges(cat-ions)
- negative charges(an-ions)
- neutral charge
A. Positive charges(+ve) known as protons, produces positive current like cations in a system. This cations losses electrons from an atom to produce positive charges and so having a relative mass of 1 (using carbon 12 as standard).
For an atom to stay stable in its physical state, it must be electrically neutral. And for it to attain that state, it must contain numerous positive charge components to balance the negative charge of the electrons in the atom.
Thomson a scientist founded protons when conducting an experiment using a cathode ray tube(discharge tube). He noticed a reddish glow opposite to the green glow and proved that the reddish glow was due to the presence of positively charge rays.
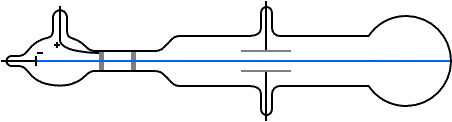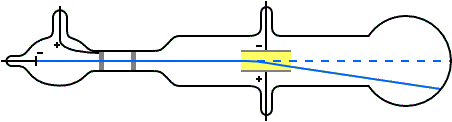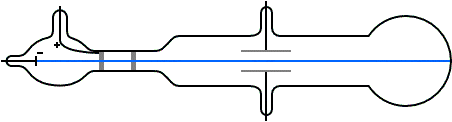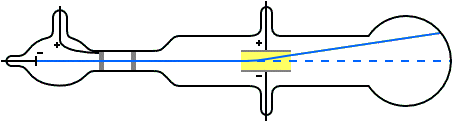
He deduced that this so called positive rays(ions) are the hydrogen ions produced by the hydrogen gas in the tube; He exclaimed that each proton carries a unit of positive charge which are heavier in mass and hence larger than the electron. They are often called helium particles or rather simply helium.
B. Negative charges(-ve) known as electrons, produces negative currents like anions in a system. This anions gain electrons in an atom to form positive charges and by so having a fluctuating relative mass.
Just as proton, JJ Thompson also discovered the existence of electron in the same experiment using the discharge tube. When he applied a potential difference of about 5000v across the tube containing a gas under low pressure of about 0.0001atm.
The tube began to glow. And when he had increased the Pd along the tube to 15000v, a very bright green glow appeared on the glass. With this he stated that the glow was caused by a kind of ray that is negatively charged which must be present in all matter.
Electrons are found to be 9.1 * 10^-11 kg , so small that they often mistook for the mass of the atom itself,hence they provide clearly the structure and shape of the given atom present in an element.
Lastly negative charges or ions are mostly called beta particles.
C. Neutral charges from the name implies are ions that are neutral. They are also sub particles called neutrons sited at the nucleus of an atom, which also exhibit the same mass as the protons do but carry no charge within them.
They were discovered by a scientist called Chadwick in 1932, when he bombarded a thin sheet of beryllium with alpha particles and discovered a new sub-particle or ion called neutrons. He concluded that this neutrons are found together with the protons in an atomic nucleus.
Do ions exist in the state of matter?
liquid state
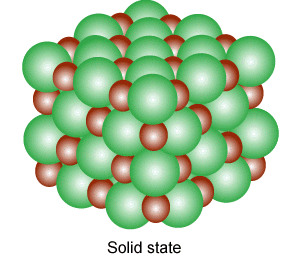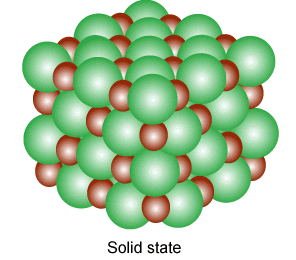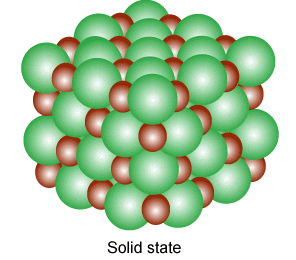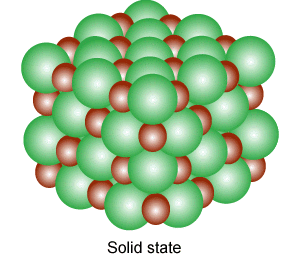
why not?
Ions exhibit such properties and also exist in various state(gaseous,liquid,solid state). A single ion is called a mono atomic ion whereas if it consists of two or more they are called molecular ions; Several molecular ions combine to form various state of matter like in gases, they are in pairs and each pair consist of free electron and positive ions.
Ions in their gaseous state are highly reactive and will react to other ions of opposite charges to form neutral molecules or ionic salts(solid state of ions).
They are produced in liquid(molten state) and solid state, when a salt is dissolved in a solvent to produce solvable ions as a result of the separating of ions to bond with the liquid particles to alter in a combination of energy and entropy.
How/why do ions bond together?
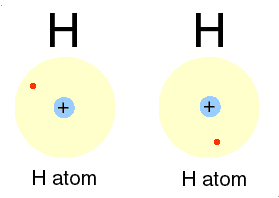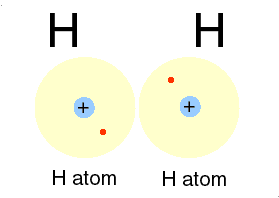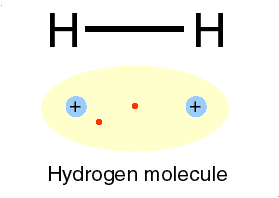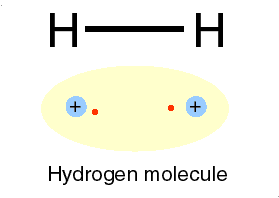
Ions bonds through the attraction and repelling process called ionic bonding. Ionic bonding is a chemical bonding that arises from the mutual attraction of opposite charged ions, since ions of like charge repels. Ions do not exist on their own as most elements do but rather they exist in their combined state forming crystal lattice(ionic compound).
Some Metals and non-metals are seen to undergo such ionic bonding except for noble gases. The ionic bonding of metals contributes to its chemical properties(being electron-positive or electron-negative).
So irrespective of its property and structure, Do ions perform any relevant function to human life?
Actually ions are applicable to all aspect of life, which are:
- In Transformers and Electric motors
- During Electrolysis
- During heating Process
- In nature
- In air Purification
- In the Human body
1.Ions in Nature:
Ions are ubiquitous in nature, hence they contribute to the luminescence of the Sun(light rays) and also to existence of the earths ionosphere.
2.Ions in Electrolysis:
Salt(solid ions) when dissolved in a solvent(water,alcohol) gives rise to liquefied ions in molten state. It acts as an electrolyte(semi-conductor) during electrolysis for chemical decomposition.
3.Ions in the Human body:
In bio-chemistry, Ions are seen to be mixed with water in the human body which contributes to the production of energy from the excess of breakdown of ATP(Adenosine Triphosphate).
4.Ions in heating process
Have you ever paced the edge of an metal on an intense flame. if yes, didn't you observe that over time the metal becomes reddish and the other edge tends to be hot.
So the question is, What really happened?
During the heating process ions are transferred from the hot flame to the metal in the form of heat(light rays) and metal being a good conductor of heat allows this ions to flow through causing a spread of ions or rather heat all around the metallic body.
THAT'S ALL FOR NOW
If you enjoyed this content and found it interesting and fun, Kindly "UPVOTE" AND "FOLLOW". Also stay tuned for more relevant contents in chemistry on my Blog.
IMAGE CREDIT
- All images were gotten from google
REFERENCE
- Wikipedia
- Chemistry Textbook for Senior Secondary Schools.
@originalworks
The @OriginalWorks bot has determined this post by @obilovejoel to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
I like your discussion there. Keep steeming :)