Physics - Faraday's laws of electrolysis
Faraday’s laws of electrolysis
The factors affecting the quantities of matter liberated during the process of electrolysis were investigated by Faraday. 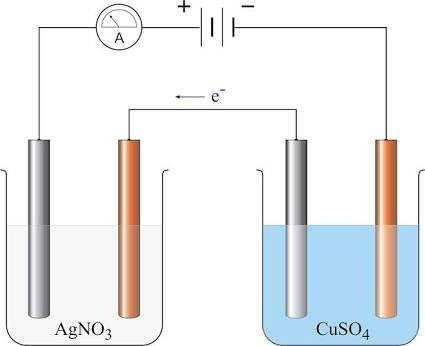
First Law :
The mass of a substance liberated at an electrode is
directly proportional to the charge passing through the electrolyte.
If an electric current I is passed through an electrolyte for a time t, the amount of charge (q) passed is I t. According to the law, mass of substance liberated (m) is
m α q or m = zIt
where Z is a constant for the substance being liberated called as electrochemical equivalent.
Its unit is kg C–1.
The electrochemical equivalent of a substance is defined as the mass of substance liberated in electrolysis when one coulomb charge is passed through the electrolyte.
Second Law :
The mass of a substance liberated at an electrode
by a given amount of charge is proportional to the chemical equivalent of the substance.
If E is the chemical equivalent of a substance, from the second law.
m α E