Synthesis and reactions of alkynes (Cracking, addition of H2O, hydrogenation and more)
Some time ago I made two articles detailing the synthesis and reactions of the alkenes, which had an acceptable support, which encouraged me to investigate to make a new article of synthesis and reactions, but this time, of the alkynes. I hope it will be you useful.
To see the previous posts check here:
Synthesis of alkynes.
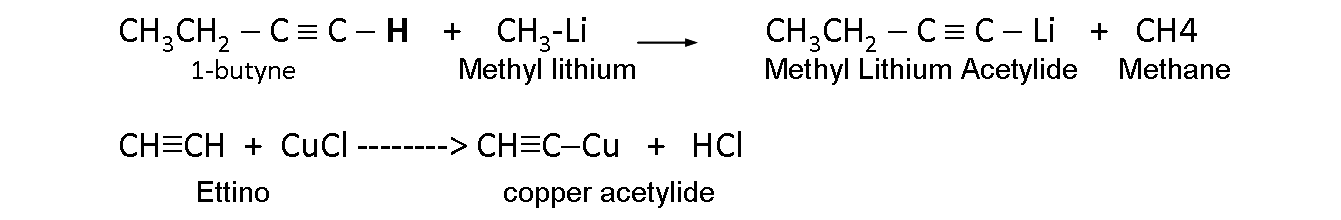
Metallic acetylides.
The alkynes with a triple bond in carbon 1º tend to lose a hydrogen. That carbon atom behaves as if it were more electronegative.
A hydrogen linked to a C 1 with triple bond can be displaced by metal atoms, forming acetylides.
The metal acetylides; they are organometallic compounds with a metal atom instead of the acetylenic hydrogen of a terminal alkyne.

Acetyl ions are carbanions that are formed from terminal acetylenes when they lose acetylenic hydrogen.
This acetylene proton is eliminated with:
- A very strong base, such as a Grignard Reagent (RMgX).
- An Organolitio (RLi).
- Sodium amide (NaNH2).
Example:
Cracking.
Cracking consists of breaking long chains, obtaining smaller ones that are more useful. It is usually carried out by means of heat or in the presence of catalysts.
Example:
Reactions of alkynes.
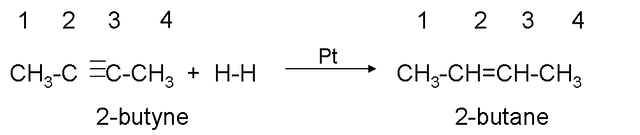
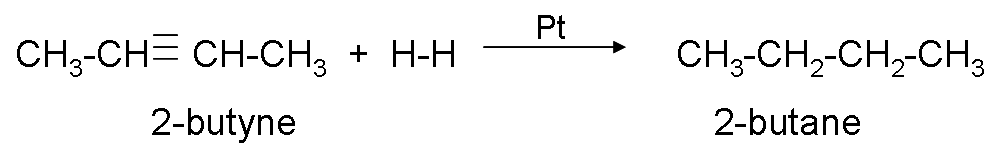
Hydrogenation.
It consists of adding hydrogen to the triple bond until it becomes a single bond, to carry out the reaction, the presence of a catalyst (platinum or nickel) is necessary.
Example:
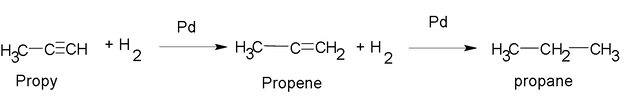
If we continue adding hydrogen to propene we obtain:
Addition of H2O.
The water additions are carried out in the presence of a mixture of catalysts composed of mercuric sulfate (HgSO4) and aqueous sulfuric acid (H2SO4). The product of the reaction follows the orientation Markovnikov.
The initial product is a vinyl alcohol, unstable, which is called enol ("in" of alkenes and "ol" by alcohol). This structure undergoes a rearrangement, which involves the loss of a proton of an oxydryl group, which is added to the adjacent carbon and the double bond is relocated, thus originating an aldehyde or ketone. This type of rearrangement is known as tautomería (in Spanish) because the product is a ketone, it is known as tautomería cetoenólica (in Spanish too).
Example:
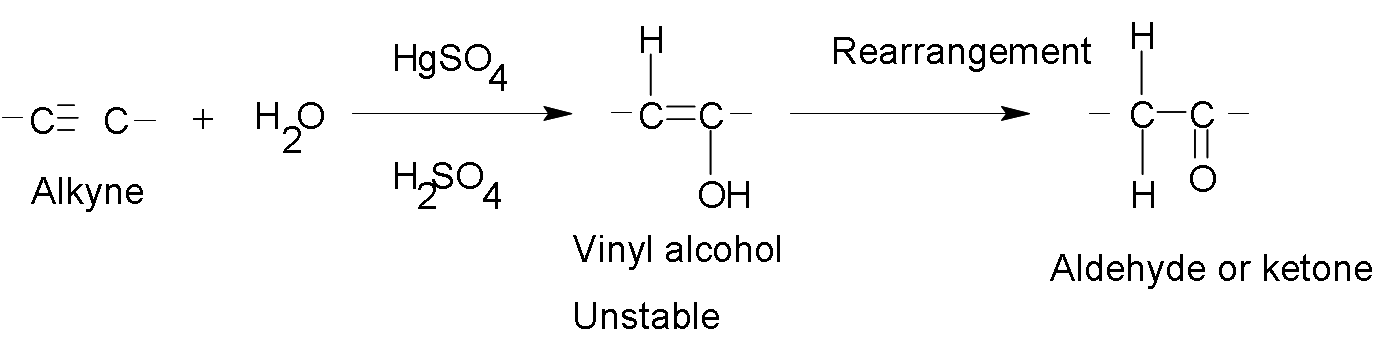
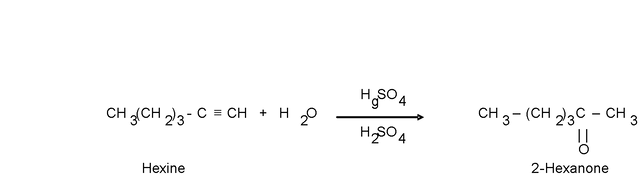
When higher terminal alkynes are hydrated, more ketones than aldehydes are formed as a product.
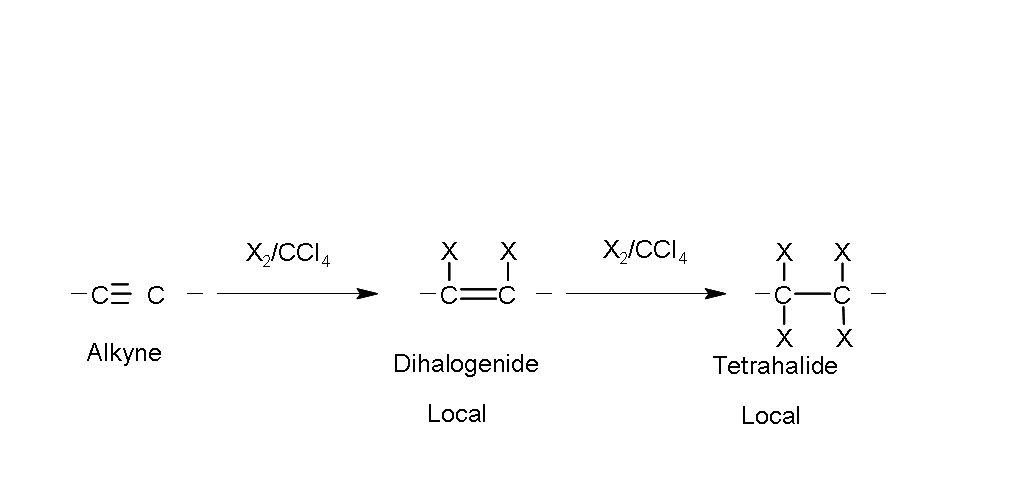
Halogenation.
The alkynes add chlorine and bromine in an inert solvent such as tetrachloride
carbon, similar to alkenes.
By adding one mole of halogen, the product is a dihaloalkene (local halide); with a second addition (two moles) a neighbor tetrahalide is obtained.
Example:
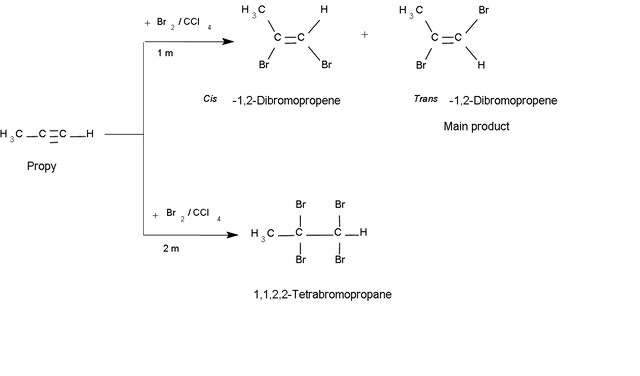
The stereochemistry of the halogen addition can be either yn (cis) or anti (trans), and the products are generally a mixture of these isomers, the main product being the anti (trans) isomer.
References:
- Organic Chemistry (5th Edition), by Leroy G. Wade.
- Modern organic chemistry, by Rodger W Griffin.
- https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtjml/addyne1.htm
- https://www.masterorganicchemistry.com/2014/01/29/synthesis-5-reactions-of-alkynes/
- http://www.organic-chemistry.org/synthesis/C3C/terminal-alkynes.shtm
- http://www.chemgapedia.de/vsengine/vlu/vsc/en/ch/2/vlu/alkine/alkinereak.vlu.html
- https://chem.libretexts.org/Core/Organic_Chemistry/Alkenes/Reactivity_of_Alkenes/Catalytic_Hydrogenation
If you want to read more scientific articles of good quality, do not waste your time, and visit the hashtag #steemstem.
Disclaimer: All the images used are correctly labeled for reuse.

Man. I can't wait till i'm a Steem millionaire
Mmm... We share ambitions.
Hello josemmr11!
Congratulations! This post has been randomly Resteemed! For a chance to get more of your content resteemed join the Steem Engine Team
Do or do not. There is no try.
Yes, you have some reason friend...
Very good your post, good information.
Thanks!