Biology Roundup #2: The week's latest discoveries in Molecular Biology.
Dear Steemians,
In last week's article we took a look at new discoveries like;
- What is the basic neurological differences between humans and non-human primates?
- How can we precision engineer genomes?
- Potential new candidates for Alzheimer's disease.
This week there are new exciting and interesting discoveries in the world of molecular biology. Scientists are hard at work exploring the limits of what genome can do and try to cure cancers; Their efforts have in some ways altered our lives. Without further adieu, let us dive into the realm of molecular biology.
Weekly round up 3 Dec 2017.
Synthetic proteins made from scratch !
It is well known that all organisms encode their proteins via DNA; Which in-turn consists of A,T,G,C as the code. A combination of these 'bases' give rise to the 20 amino acids the living cells use to make the proteins.
A team led by chemist Floyd Romesberg, Have come up with two more alphabets in the genetic code. They have improved upon an earlier report wherein a strain of E.Coli had a plasmid with unnatural base pair dNAM-dTPT3. The initial report was a proof of concept wherein the bacteria were sluggish and failed to replicate efficiently. Here they report successful in-vitro transcription of DNA containing synthetic bases. The incorporation leads to the production of two new amino acids PrK and pAzF into a protein that emits a soft green glow.
This fascinating synthetic organism has potential to fight antibiotic resistance, create new peptide-based drugs and the possibilities are yet to be looked into.
Check the original article and accompained commentary on Nature.
Friends in war: CD27 agonist One-upping antibody therapy for cancers.
The immune system is known to all of us to attack the rat-shit (pardon the lingo) out of anything foreign to the body. This has been previously exploited to create cancer drugs. Cancer immunotherapy uses antibodies against specific proteins expressed by cancer cells to help identify the immune system and attack them. However few mechanistic details on how to make this robust have remained elusive.
In the article by Anna H. Turaj and colleagues, They solve this question by using an anti-CD20 antibody; a well known also as rituximab for treating B cell lymphomas and immuno-modulatory antibody anti-CD27 to decimate cancer.
Using single-cell RNA sequencing they demonstrate the mechanisms by which this treatment is effective in mouse models. It seems to activate the myeloid cell infiltration and macrophage activation. In theory, the body recognizes the cancer cells and selectively destroys them.
Source of the article : Cancer Cell
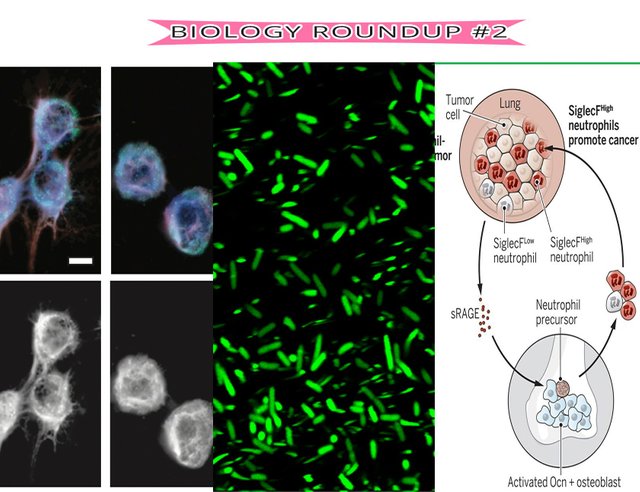
Complicated relationship: Are Bone cells helping lung tumours?
Yes, they do says the latest article on science. The researchers have long known that there is some connection with regards to lung tumors and bone metastasis; but they have finally figured out how and why.
A group of cells known as myeloid cells are key regulators of cancer growth. They are abundant in cancers and their association is often seen as 'dangerous' for cancer outcomes. These cells originate at distant tissues; One such is the bone marrow which produces hematopoietic cells. Bone also consists of various other cell types, such as osteoblasts; these cells participate in regulation of hematopoesis and immune responses. Their role in aiding and abbeting in cancer had remained un-explored.
In this report by Camilla Engblom and co-workers; They have systematically shown.
- Osteocalcin-expressing osteoblastic cells affect distant tumor progression.
- They allow infiltration of a subset of neutrophils called SiglecF_high; These inturn express genes promoting cancer processes like angiogenesis, suppression of T cell responses and ECM remodelling to name a few.
- A particular receptor named sRAGE is upregulated in mice bearing tumors and fosters osteoblastic activity.
In conclusion, they identfy an unique crosstalk between two cell types and describe for the first time how bones/osteoblastic cells provide for progression of cancer. Now it can be used as a bio-marker and potential target for anticancer therapy.
This work has been published in Science. A simplified version can be accesed here
Old Proteins & New Functions
Researcher Arturo Zyclinsky and his team have come up with an interesting paper last week. The paper titled "Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps" shows how cyclin dependent kinases whose functions are generally associated with progression of cell cycle have an alternate function. They seem to act in coordination withspecificc mitogenic stimuli and trigger the formation of NET's. The formation of NET's is a recent discovery where neutrophils throw out a mesh of DNA to trap any pathogens. Here they show that CDK4/6 is absolutely essential for the clearance.
The paper is accessible in Developmental cell
To Be continued !
I hope you have liked this week's roundup. It has been an immunology intense week, I am trying to put up more articles in the roundup. I hope to increase it to 5 articles next week. Please feel free to contact me or comment re-steem it. It helps to spread the word out in the community.

Image Sources:
All the images used are attributed to the corresponding authors. They are not mine. Please take time to check their galleries as it gives them more exposure and you will get to know their work better.
GFP E-Coli : iGEM
Bone Pathway: Science
NET: Developmental cell
Awesome to see the protein expressed and the bacteria healthy.
Synthetic proteins made from scratch thou ?
Hardly semi syntheic and even if all amino acids where synthetic the "protein" would be a worthless peptide, how could it fold into anything usable?
This is simply a mutated DNA expressed as 2 new amino acids,a new type of mutation, which is cool but how does it help creating new proteins and cures ? if anything it should make the simulation of folding even harder!
Synthetic peptides is easy to make and nothing new, these new aa´s could easly be incorporated if they had any purpose. The peptides just wont fold into proteins and new aa´s wont make them fold any better!
Did the superfolder GPF even fold ? did it function ? i wounder, bleh 5 dollar for the article and it wont even say!
Cool post! molecular biology relating to the genome is definitely a huge area where R and D needs to continue! The next big move in health is precision medicine, so we can ultimately try to eliminate some of these horrible diseases which take so many lives. Thanks for sharing !!
Thank you for reading :). Yes, precision medicine is definitely starting to gather attention.
You got a 43.46% upvote from @upme thanks to @harshameghadri!
Hi. My friend is like you. She lost 60 steem. @linhlinhvictoria .Do you have to steady your password?
Yes, I recovered my account and changed my password @a-alice
Congratulations @harshameghadri, this post is the sixth most rewarded post (based on pending payouts) in the last 12 hours written by a User account holder (accounts that hold between 0.1 and 1.0 Mega Vests). The total number of posts by User account holders during this period was 2309 and the total pending payments to posts in this category was $2431.92. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.