Physics of Fluids: The Leidenfrost Effect or How to Make a Liquid Levitate (Scientific Experiment)
Hi Dear Steemians! Today I will have a little experience, and I will also tell you about a very interesting phenomenon in physics. After all, for sure, many people in their kitchen had to see how a drop of liquid falling on a very hot pan does not evaporate immediately, but for a minute or even longer they spin on its surface (then the drop naturally evaporates). Surprising is not it?
Such an abnormally long "life" of a drop in physics is referred to as the - Leidenfrost effect. The effect itself was named in honor of the German physicist Johann Gottlob Leidenfrost who described this phenomenon in the distant 1750s. The essence of this effect is that the liquid on contact with the body, much hotter than the boiling point, creates an insulating vapor layer that protects the liquid from rapid boiling and prevents strong heating. To understand how this happens visually, look at the images below.
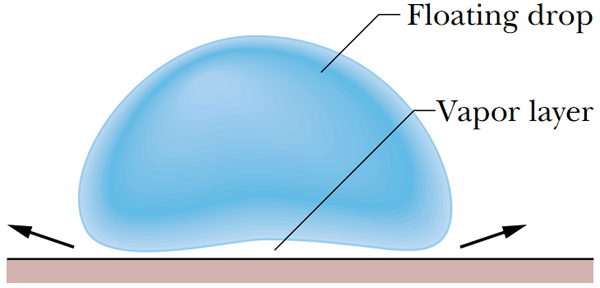
A drop of liquid on a hot surface, thanks to a thin heat-insulating vapor layer, does not immediately evaporate, but continues to exist for a fairly long time, levitating above the substrate. The arrows show the direction of the steam flow. Image from the article J.Walker - Boiling and Leidenfrost effect.
Proceeding from this, it can be concluded that the drop due to a peculiar "air cushion" significantly prolongs its "life" levitating over the hot surface. In order to understand how this happens, we can consider a graph showing us the "life" time of a drop of water, depending on the temperature of the substrate. And in order to understand how this happens, we will not consider boring graphics, but we will conduct a small experiment.
To repeat, the fact that in the scientific world is called the Leidenfrost effect, we first need an empty pan, which we put on the fire and will be heated for two minutes. Further for better effect, we need distilled water (the purer the liquid and the better the surface quality, the longer our droplets will live), which must be poured into the heated pan, and then wait until all the water in the pan has evaporated completely.

After the water in the pan evaporates, the empty pan again needs to be heated for five minutes - this is done in order to form the heat-insulating surface mentioned above. And then we take the tube and start to drip into the pan droplets of ordinary water. And what do we see?
Water does not evaporate! It only disperses into small droplets, and then a large drop merges again into the shape. At some point, I even had a feeling that water begins to behave like mercury or be in space without the force of friction. But we'll go a little further and refine our experiment.
For this, I'll take an ultraviolet lamp and text highlighters that glow under UV light - they were bought in an ordinary stationery store. Then we'll have to get the core out of the text divider, cut it in half and put it in the water until it's painted. As a result, I got such a cool luminous liquid.

And now I turn on the ultraviolet lamp and the experiment looks quite different:
Well, now you can go to the theoretical part - why does not the drops evaporate? Here everything is very simple - while the temperature of the pan is less than 100 ° C, the lifetime of the droplets will decrease and reach a speed of 200 m / s at the limit point (the boiling point of water). At this temperature, our droplets come in contact with the surface and begin to boil. And as soon as the surface temperature reaches 100-150 ° C, the lifetime of the drop due to the formation of an insulating vapor layer begins to increase rapidly. The maximum lifetime of the drop is determined by the so-called Leidenfrost temperature, which is individual for each liquid.
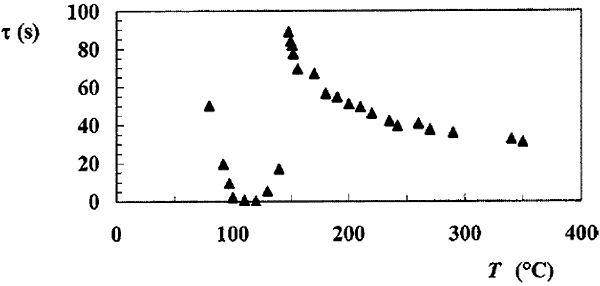
The lifetime of a drop of water with a radius of 1 mm, depending on the temperature of the duralumin surface on which it is located. Image from the article Anne-Laure Biance - Leidenfrost drops.
But for what is it used by a phenomenon that nobody needs at first glance?
In recent years, the study of the dynamic characteristics of drops of various liquids attracts more and more scientists. In part, this interest is due to the advent of high-speed video cameras capable of capturing to the smallest detail the behavior of droplets on the surface with given physical and chemical parameters, their collision, and the interaction of drops with various bodies and media. The analysis of these video data allows, first, to check the existing theoretical models describing these phenomena, and secondly, to reveal any new facts not predicted by theories. The study of droplet dynamics is also of great practical importance: its results are used in lab-on-a-chip, inkjet, spraying and even in such a mundane process as painting.
Although some people use this effect not for their intended purpose, in general, see the video below.
Well, now if you've carefully read everything, answer me a couple of questions - why did not the molten aluminum stick to the steak? And why did not the steak completely be cooked? And while you answer these questions, I am going to prepare the following material for you. See you next time :)
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by floxxy from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
@resteemator is a new bot casting votes for its followers. Follow @resteemator and vote this comment to increase your chance to be voted in the future!