Ozone
( )
)
Ozone or tri-oxygen, is an inorganic molecule with the chemical formula O3. It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O2, breaking down in the lower atmosphere to O2 or dioxygen.
Ozone is formed from dioxygen by the action of ultraviolet light and also atmospheric electrical discharges, and is present in very low concentrations throughout the Earth's atmosphere (stratosphere). Its concentration is highest in the ozone layer region of the atmosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.
Ozone's odour is sharp, reminiscent of chlorine, and detectable by many people at concentrations of as little as 100 ppb in air. Ozone's O3 structure was determined in 1865. The molecule was later proven to have a bent structure and to be diamagnetic. In standard conditions, ozone is a pale blue gas that condenses at progressively cryogenic temperatures to a dark blue liquid and finally a violet-black solid. Ozone's instability with regard to more common dioxygen is such that both concentrated gas and liquid ozone may decompose explosively at elevated temperatures or fast warming to the boiling point. It is therefore used commercially only in low concentrations.
Ozone is a powerful oxidant (far more so than dioxygen) and has many industrial and consumer applications related to oxidation. This same high oxidising potential, however, causes ozone to damage mucous and respiratory tissues in animals, and also tissues in plants, above concentrations of about 100 ppb. This makes ozone a potent respiratory hazard and pollutant near ground level. However, the ozone layer (a portion of the stratosphere with a higher concentration of ozone, from two to eight ppm) is beneficial, preventing damaging ultraviolet light from reaching the Earth's surface, to the benefit of both plants and animals.
Location and Production

The highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above the surface (or between about 6 and 31 miles). However, even in this layer, the ozone concentrations are only two to eight parts per million, so most of the oxygen there is dioxygen, O2, at about 210,000 parts per million by volume.
Ozone in the stratosphere is mostly produced from short-wave ultraviolet rays between 240 and 160 nm. Oxygen starts to absorb weakly at 240 nm in the Herzberg bands, but most of the oxygen is dissociated by absorption in the strong Schumann–Runge bands between 200 and 160 nm where ozone does not absorb. While shorter wavelength light, extending to even the X-Ray limit, is energetic enough to dissociate molecular oxygen, there is relatively little of it, and, the strong solar emission at Lyman-alpha, 121 nm, falls at a point where molecular oxygen absorption is a minimum.
The process of ozone creation and destruction is called the Chapman cycle and starts with the photolysis of molecular oxygen;
O2 + photon (radiation λ < 240 nm) → 2 O
followed by reaction of the oxygen atom with another molecule of oxygen to form ozone.
O + O2 + M → O3 + M
where "M" denotes the third body that carries off the excess energy of the reaction. The ozone molecule can then absorb a UV-C photon and dissociate
O3 → O + O2 + kinetic energy
The excess kinetic energy heats the stratosphere when the O atoms and the molecular oxygen fly apart and collide with other molecules. This conversion of UV light into kinetic energy warms the stratosphere. The oxygen atoms produced in the photolysis of ozone then react back with other oxygen molecule as in the previous step to form more ozone. In the clear atmosphere, with only nitrogen and oxygen, ozone can react with the atomic oxygen to form two molecules of
O3 + O → 2 O2
Physical properties
Ozone is colorless or slightly bluish gas (blue when liquefied), slightly soluble in water and much more soluble in inert non-polar solvents such as carbon tetrachloride or fluorocarbons, where it forms a blue solution. At 161 K (−112 °C; −170 °F), it condenses to form a dark blue liquid. It is dangerous to allow this liquid to warm to its boiling point, because both concentrated gaseous ozone and liquid ozone can detonate. At temperatures below 80 K (−193.2 °C; −315.7 °F), it forms a violet-black solid.
Most people can detect about 0.01 μmol/mol of ozone in air where it has a very specific sharp odor somewhat resembling chlorine bleach. Exposure of 0.1 to 1 μmol/mol produces headaches, burning eyes and irritation to the respiratory passages. Even low concentrations of ozone in air are very destructive to organic materials such as latex, plastics and animal lung tissue.
Ozone is diamagnetic, which means that its electrons are all paired. In contrast, O2 is paramagnetic, containing two unpaired electrons.
Structure
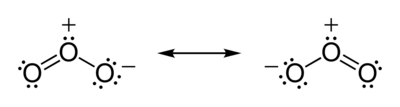
According to experimental evidence from microwave spectroscopy, ozone is a bent molecule, similar to the water molecule. The O – O distances are 127.2 pm (1.272 Å). The O – O – O angle is 116.78°. The central atom is sp² hybridized with one lone pair. Ozone is a polar molecule with a dipole moment of 0.53 D. The molecule can be represented as a resonance hybrid with two contributing structures, each with a single bond on one side and double bond on the other. The arrangement possesses an overall bond order of 1.5 for both sides.
Reactions
Ozone is among the most powerful oxidizing agents known, far stronger than O2. It is also unstable at high concentrations, decaying to ordinary diatomic oxygen. It has a varying half-life length, depending upon atmospheric conditions (temperature, humidity, and air movement). In a sealed chamber with a fan that moves the gas, ozone has a half-life of approximately a day at room temperature. Some unverified claims imply that ozone can have a half life as short as a half an hour under atmospheric conditions.
2 O3 → 3 O2
This reaction proceeds more rapidly with increasing temperature and increased pressure. Deflagration of ozone can be triggered by a spark, and can occur in ozone concentrations of 10 wt% or higher.
Ozone can also be produced electrochemically at the anode of an electrochemical cell from oxygen. This reaction can be used to create smaller quantities of ozone for research purposes.
O3(g) + 2H+ + 2e− ←→ O2(g) + H2O E°= 2.075V
This reaction can be observed as an unwanted reaction in a Hoffman gas apparatus during the electrolysis of water when the voltage is set above the necessary voltage.
With metals
Ozone will oxidise most metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state. For example:
Cu + O3 → CuO + O2
With sulfur compounds
Ozone oxidises sulfides to sulfates. For example, lead(II) sulfide is oxidised to lead(II) sulfate:
PbS + 4 O3 → PbSO4 + 4 O2
Sulfuric acid can be produced from ozone, water and either elemental sulfur or sulfur dioxide:
S + H2O + O3 → H2SO4
3 SO2 + 3 H2O + O3 → 3 H2SO4
In the gas phase, ozone reacts with hydrogen sulfide to form sulfur dioxide:
H2S + O3 → SO2 + H2O
In an aqueous solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce sulfuric acid:
H2S + O3 → S + O2 + H2O
3 H2S + 4 O3 → 3 H2SO4
Ozone Cracking

Ozone gas attacks any polymer possessing olefinic or double bonds within its chain structure, such as natural rubber, nitrile rubber, and styrene-butadiene rubber. Products made using these polymers are especially susceptible to attack, which causes cracks to grow longer and deeper with time, the rate of crack growth depending on the load carried by the rubber component and the concentration of ozone in the atmosphere. Such materials can be protected by adding antiozonants, such as waxes, which bond to the surface to create a protective film or blend with the material and provide long term protection. Ozone cracking used to be a serious problem in car tires for example, but the problem is now seen only in very old tires.[clarification needed][citation needed] On the other hand, many critical products, like gaskets and O-rings, may be attacked by ozone produced within compressed air systems. Fuel lines made of reinforced rubber are also susceptible to attack, especially within the engine compartment, where some ozone is produced by electrical components. Storing rubber products in close proximity to a DC electric motor can accelerate ozone cracking. The commutator of the motor generates sparks which in turn produce ozone.
References
https://en.wikipedia.org/wiki/Ozone
https://www.google.com.ng/search?q=images+og+ozone&oq=images+og+ozone&aqs=chrome..69i57.5121j0j7&sourceid=chrome&ie=UTF-8