THE CHEMISTRY OF COLOUR: Colour in Azo Compounds and How Dyes are Stuck onto Textiles.
The first commercially successful azo dye was
Chrysoidine. The azo group, – N=N –, acts as a ‘delocalisation bridge’ between
the two benzene rings to form an extended delocalised system, which is the
chromophore. The two amine functional groups of chrysoidine have lone pairs of
electrons on the nitrogen atoms. These lone pairs interact with the delocalised
system.
The nature of the functional groups that interact with the chromophore can dramatically alter the colour of the azo dye molecule by causing a shift in the electrons of the chromophore. This electron shift alters the energy required to promote them into an excited state, and so shifts the wavelength of light absorbed.

How are dyes stuck onto textiles?
The dye of blue denim Jeans is indigo and it is called a vat dye. A vat dye is usually soluble in its reduced form, but when oxidized becomes insoluble and precipitates in the pores of the denim cotton fibres. This property means that vat dyes do not wash out of clothes. (To learn a little more about the history of blue denim and indigo, read the first two posts I wrote on colour here and here)
Vat dyes are particularly effective for cotton fibres and other fabrics that contain cellulose. The large number of hydroxyl groups on the cellulose molecules mean that the fabric readily absorbs water and hence the water-soluble dye. This is due to the formation of hydrogen bonds between water molecules and the hydroxyl groups.
Another type of cotton dye is called a direct dye. Direct dyes are long planar molecules that can lie alongside the cellulose polymer chains, and form intermolecular hydrogen bonds and induced dipole-induced dipole forces. Such large dye molecules are made water-soluble using SO3- Na+ groups. Direct dyes are usually diazo or triazo dyes.
The intermolecular forces between direct dyes and cotton are relatively weak, so the water-fastness of the dye is poor and some of the dye comes out when the material is washed. However, in 1956 chemists at ICI produced a dye that would form covalent bonds with the cellulose fibres of cotton. The covalent bonds are formed by reactive groups that first bond to the dye molecules. These groups then react with the hydroxyl groups on the cellulose fibres. The dye is water-fast and the colour does not run when the textile is washed because covalent bonds have formed with the cellulose fibres, Such a dye is called fibre-reactive.
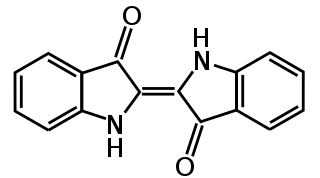
Vat Blue 1. Yikrazuul, Public Domain
Wool and silk contain many amino functional groups (NH2). These groups are basic and react with acid dyes that contain sulfonic acid groups (SO3H) to form ionic bonds.
Poly(propenenitrile) fibres (acrylics) contain CO2H and SO3H groups, which are acidic. These can form ionic bonds with basic dyes. The last group of dyes I consider are disperse dyes. All the other dye groups are water-soluble when applied, but polyester fibres do not form hydrogen bonds with water and are known as hydrophobic (water-hating). They do not allow water molecules to penetrate them. Disperse dyes are a fine suspension of dye particles that are absorbed by the fibres and held there by induced dipole-induced dipole forces and some hydrogen bonding. As the dye is only sparingly soluble it tends to stay in the fibres, and so it is water-fast.
pH INDICATORS
Methyl orange is an azo compound that has different colours depending on the pH of the solution it is in. This property means that it is used as an indicator in acid-base reactions. Adding or removing an H+ ion causes an electron shift in the molecule, and so alters the wavelength at which methyl orange absorbs. Indicators do change colour depending on the concentration of H+(aq) ions in the solution. Each indicator changes colour at a specific pH; using the correct indicator, any neutralisation reaction can be monitored to its end point. Methyl orange changes colour between pH 3.2 and pH 4.4. It can be used to determine the neutralisation point of a strong acid and a weak base.
Phenolphthalein is colourless below pH 8.2, but above this it changes to purple. This indicator can be used in titrations with strong alkalis and weak acids.
Ultraviolet and visible spectroscopy
There are two types of spectroscopy that involve absorption of electromagnetic radiation by a substance under investigation. In infrared spectroscopy, light is absorbed in the infrared part of the spectrum because of the increased vibration of different bonds within a molecule. In nuclear magnetic resonance spectroscopy, radio waves are absorbed through the excitation of nuclei within molecules,
Ultraviolet and visible spectroscopy are possible because outer electrons of atoms or ions in compounds absorb in the ultraviolet or visible part of the spectrum when they are excited. Compounds that absorb only in the ultraviolet part of the spectrum are colourless. In the spectrometer, a beam of electromagnetic radiation passes through a monochromator, which selects varying wavelengths. The beam is then split and one beam passes through a solution of the substance under investigation, while the other passes through the pure solvent.
The spectra produced usually have broad absorption bands, in contrast to atomic absorption spectra in which gaseous atoms and ions have definite sharp lines. The broad absorption bands occur because in solution a number of vibrational and rotational energy levels are possible for each energy level of the electrons.
INTERPRETING ULTRAVIOLET-VISIBLE ABSORPTION SPECTRA
The horizontal axis of an ultraviolet-visible absorption spectrum gives the wavelength in nanometres (nm). The shape of the absorption peak is usually characteristic of a particular compound and so the spectrum can be used to help identify the compound.
However, a more common use of this type of spectroscopy is to measure concentrations accurately from the intensity of absorption on the y-axis. For example, the uptake of a drug at different sites around the body can be monitored using ultraviolet-visible spectroscopy by taking samples from these sites and analysing their solutions.
In the steel industry, the amount of trace metal, such as manganese, can be analysed by reacting the metal to form an identifiable coloured ion and measuring the absorption of its solution. In this case, the manganese may be oxidised to form the purple MnO4- ion.
The food industry also uses ultraviolet-visible spectroscopy to determine how much nitrite has been added to meat.
TRANSITION METAL IONS AND COLOUR
A characteristic of transition metals is that many of their compounds are coloured. Transition metal compounds are responsible for the colours in gemstones, stained glass windows and pottery glazes. From reading this topic, you will realize that transition metal ions appear coloured because they absorb some wavelengths in the visible spectrum, but transmit or reflect the rest. The electrons absorb photons of a specific wavelength, become excited and jump to higher energy levels. In compounds of transition elements, colour results from a difference in energies between d orbitals.
d-d TRANSITIONS
You are probably wondering how d orbitals can be at different energy levels. d orbitals were drawn at the same energy level (degenerate) on energy level diagrams. However, this is only true for gaseous transition metal ions. When ligands bond to transition metal ions they cause a splitting of the energy level of the d orbitals. The energy difference between the two sets of d orbitals is often such that the wavelength of photons absorbed is in the coloured part of the spectrum.
Copper(I) compounds are white because the Cu+ ion has a 3d10 outer electron configuration. Since the 3d subshell is full, no transition of electrons between d orbitals can occur. The same is true of scandium(III) compounds which have no electrons in the 3d subshell (3d°). But not all colour in transition metals results from d-d transitions: sometimes, electrons can jump from the ligand to the metal. This is called charge transfer or electron transfer, and it is responsible for the bright colours of Prussian blue and chrome yellow.

FACTORS THAT AFFECT d-d SPLITTING AND COLOUR
The colour of a transition metal complex depends chiefly on the central metal cation. Each transition metal is different and has a different nuclear charge. The larger the nuclear charge, the more firmly electrons are held in their d orbitals. This affects the energy levels of the split d orbitals and thus the amount of energy required to excite an electron from a lower energy d orbital to a higher energy d orbital. This in turn affects the colour.
The oxidation state of the metal also affects the splitting of the d orbitals and, as a result, the colour. This is well illustrated by vanadium complexes in aqueous solution.
The nature of the ligand also has an effect on d-d splitting. Different ligands cause different separations of energy between d orbitals. The spectrochemical series is a list of ligands arranged in order of their ability to cause d-d splitting:
I- < Br- < Cl- < F- < OH- < H2O < (CO2)2- < NH3 < en < CN-
smallest splitting to the greatest splitting
smallest energy gap to the greatest energy gap
longest wavelength to the shortest wavelength
[Cu(H2O)6]2+ absorbs at the red end of the spectrum, which makes an aqueous solution of copper(II) ions appear pale blue. However, by substituting H2O with NH3 ligands to form the ion [Cu(NH3)4(H2O)2]2+, the difference in energy of the d orbital split increases.
This shifts the absorption into the yellow part of the spectrum, which makes the solution deep blue.
Another factor that has an effect on d-d splitting is the number of each type of ligand in the complex: [Cr(H2O)6]3+ is violet, [Cr(H2O)5Cl]2+ is green and [Cr(H2O)4Cl2]+ is dark green. The arrangement of ligands around the central metal cation can also have an effect on the colour of a complex.
REFERENCES
https://en.wikipedia.org/wiki/Azo_dye
https://en.wikipedia.org/wiki/Azo_compound
https://www.sciencedirect.com/topics/chemistry/azo-compound
https://www.britannica.com/topic/textile/Dyeing-and-printing
https://www.quora.com/What-makes-clothing-dye-stay-in-fabrics
https://www.moneycrashers.com/how-to-dye-fabric-clothes-make-natural-dyes/
https://en.wikipedia.org/wiki/Disperse_dye
https://www.chemicalbook.com/ProductCatalog_EN/161113.htm
https://www.thesprucecrafts.com/fiber-reactive-dye-1106363
https://www.britannica.com/technology/direct-dye
https://textilelearner.blogspot.com/2011/02/defination-classification-application_2111.html
https://en.wikipedia.org/wiki/Vat_dye
https://www.compoundchem.com/2014/04/04/the-colours-chemistry-of-ph-indicators/
https://www.thoughtco.com/definition-of-ph-indicator-605499
https://en.wikipedia.org/wiki/PH_indicator
https://www.jove.com/science-education/10204/ultraviolet-visible-uv-vis-spectroscopy
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.