ALKENES: The Preparation, Properties, Reactions and Tests.
The alkenes form a homologous series. The first three members are gases, but all the higher members are liquids or solids. The increase in the boiling point of compounds as the number of carbon atoms per molecule increases is typical for all homologous series, not just the alkenes.
PREPARATION OF ALKENES
CRACKING ALKANES
Saturated hydrocarbons can be cracked to give alkenes. Though cracking can be carried out in the laboratory, this method is unsuitable because the mixture it produces is difficult to separate into individual alkenes.
Free-radical mechanism of cracking
Thermal cracking involves heating long chain alkanes at a very high temperature so that the molecules break down to give hydrocarbons with shorter carbon chains. Since the C-C bond in alkanes is non-polar, the breaking of this bond is believed to involve homolytic fission with the formation of alkyl free radicals. Homolytic fission is normally initiated by ultraviolet light, but a very high temperature provides sufficient thermal energy for the process.
Cracking lacks selectivity, since its progress is determined by which C-C bond is first broken, and then by the type of collision in which the free radical is involved. Typically, the cracking of a hydrocarbon such as decane leads to a collection of short-chain alkanes, alkenes and hydrogen.
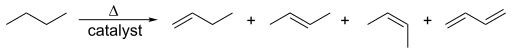
Dehydrogenation of butane to give butadiene and isomers of butene. Hbf878, CC0
DEHYDRATION OF ALCOHOLS
A suitable way to prepare alkenes is to dehydrate an alcohol. This reaction is an example of elimination and involves the loss of a molecule of water. Normally, an acidic catalyst, such as concentrated phosphoric (V) acid or concentrated sulphuric acid, is used to aid elimination of the water. Industrially, the dehydration is usually catalyzed by passing the alcohol vapour over heated aluminium oxide.
Sometimes, the dehydration of an alcohol leads to the formation of two or more alkenes, which can be either structural or geometrie isomers.
Normally, the most stable alkene is produced in a greater proportion than the other isomers. This is the alkene with most alkyl groups attached to the carbon atoms that form the double bond (the most substituted alkene).
ELIMINATION OF HYDROGEN HALIDES FROM HALOGENOALKANES
When a halogenoalkane is heated with a strong base, such as ethanolic sodium hydroxide, the corresponding alkene is produced. Again, the reaction may give more than one alkene and may be complicated by substitution products as well.
REACTIONS OF ALKENES
The double bond in an alkene is non-polar, but it is electron rich because of the π bond. When the π bond is broken, the carbon atoms remain joined together by the σ bond.
ELECTROPHILES
An electrophile is a particle that can accept a pair of electrons to make a covalent bond. Often, an electrophile has only six electrons in its outer shell, so that by accepting two others it achieves a stable octet. A typical electrophile is Cl+, with only six electrons in its outer shell. Metai ions are not electrophiles because, although they are positive, they cannot gain a pair of electrons to make a covalent bond. In fact, a metal ion is often positively charged to preserve a stable octet of electrons.
Electrophiles are generated by the heterolytic fission of a covalent bond. In heterolytic fission, the bond is broken as both bonding electrons migrate to the atom that is more electronegative, leaving the less electronegative atom positive. In the heterolytic fission of a covalent bond, we follow the convention of using a curly arrow to show the migration of a pair of electrons.
ELECTROPHILIC ADDITION
Almost all double bonds will undergo a reaction known as addition. During addition, two substances react together to give one product and the original double bond becomes a single bond. In the case of the C-C bond, an electrophile is often needed to break the bond. Since the initial step in the mechanism involves an electrophile, it is called electrophilic addition.
Mechanism of electrophilic addition
The first stage in electrophilic addition is the formation of the electrophile. The electrophile ‘attacks’ the double bond. At the same time, a pair of electrons migrates from the double bond towards the electrophile. The effect of this is to break the carbon-carbon double bond and to make a single bond to the electrophile. In the final stage, the nucleophile reacts with the carbocation that has been formed, to complete the addition.
The net effect is to add atoms at the double bond to form a saturated ompound. It is this ability to react by addition that accounts for the difference in reactivity between an alkane and an alkene.
ADDITION OF HYDROGEN HALIDES
Hydrogen bromide reacts with alkenes to produce bromoalkanes. The other hydrogen halides react with alkenes in a similar way. Hydrogen halides act as electrophiles, because the hydrogen end of the molecule is electron deficient as a result of the difference in electronegativity between the hydrogen and halogen atoms. The hydrogen end of the molecule can accept an electron pair while simultaneously the hydrogen-halogen bond is broken. The reaction with hydrogen iodide is particularly favoured because of the relatively low bond energy of the H-I bond.
Ethene reacts with hydrogen chloride to give chloroethane. Likewise, both cis- and trans-but-2-ene yield 2-bromobutane. It does not matter what the starting isomer is, because after the addition the saturated product has acquired a single bond that can freely rotate, with loss of the cis-trans isomerism. In fact, two products are formed, which in this case are optical isomers.
The production of many petrochemicals starts with this type of electrophile addition to give a halogenoalkane. The introduction of the carbon-halogen bond offers further possibilities for organic synthesis.
Addition of hydrogen halides to unsymmetric alkenes: Markovnikov's rule
An unsymmetric alkene, such as propene, is an alkene that has different groups attached to the carbon atoms of the C=C bond. The electrophilic addition of a hydrogen halide to an unsymmetric alkene can give more than one addition product, according to which end of the double bond the electrophile attacks. For example, the electrophilic addition of hydrogen bromide to propene can yield two products, 1-bromopropane and 2-bromopropane, depending on which end of the double bond is attacked by the electrophile (essentially a proton). The two products arise from different carbocations.
A primary carbocation is a species in which a positive carbon atom is attached to just one other carbon atom (of the allkyl or aryl R group). It is much less stable than a secondary carbocation, which is much less stable than a tertiary carbocation. The source of this stability is the alkyl or aryl group, which tends to donate electron density to the positive carbon atom and thereby stabilises the positive charge. It follows that two alkyl (or aryl) groups give more stability than one, and three give more stability than two. This action of pushing the electron density is called an inductive effect. The major product is formed from the most stable carbocation. Therefore, in the example of the addition of hydrogen bromide to propene, the major product is 2-bromopropane.
Markovnikov's rule predicts the way that hydrogen halides are added to unsymmetric alkenes: An electrophile adds to an unsymmetric alkene so that the most stable carbocation is formed as an intermediate.
The term 'electrophile’ is used to express the general concept, although the electrophile is often an electron-deficient hydrogen atom or a proton.
HYDRATION OF ALKENES
The hydration of an alkene is the electrophilic addition of water to give an alcohol. This reaction needs an acidic catalyst, since water itself is not a good electrophile. In the laboratory, sulphuric acid is used as the catalyst. With reactive double bonds, dilute acid is sufficient. but with alkenes, such as propene and ethene, concentrated acid is required.
In the use of concentrated sulphuric acid as a catalyst. The proton transferred by the acid is needed to start the reaction as it facilitates the breaking of the double bond. At the end of the reaction, the acid is regenerated.
In the petrochemical industry, alkenes are hydrated to give several important solvents. Hydration is achieved either by the catalytic addition of water as steam to the alkene at 7000 kPa (70 atmospheres) pressure at 300 °C, using H3PO4 as a catalyst, or by using the concentrated sulphuric acid route just described. Ethene is converted into ethanol, propene into propan-2-ol, and but-1-ene into butan-2-ol. All three alcohols are extremely useful industrial solvents.
REACTION WITH HALOGENS
Halogens can add to double bonds to give dihalogenoalkanes. For example, bromine reacts with propene to give 1,2-dibromopropane:
CH3CH=CH2 + Br2 → CH3CHBrCH2Br
Mechanism of the reaction
Compared with a hydrogen halide, it is much more difficult to visualise a bromine molecule as an electrophile, since it is not a polar molecule and both of its atoms have a stable octet of electrons. Nevertheless, a bromine molecule is able to accept an electron pair, provided that, at the same time, the bromine-bromine single bond is broken by heterolytic fission.
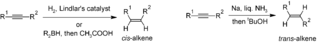
Synthesis of cis- and trans-alkenes from alkynes. Walkerma, Public Domain
As the bromine molecule approaches the double bond, the pair of electrons in the Br-Br bond moves to one of the bromine atoms. Why should this happen? After all, there is no positive or negative end to the bromine molecule, as there is in the addition of hydrogen bromide. The reason is that a temporary dipole is created in the bromine molecule as it approaches the π electrons of the double bond. It is as though the electrons in the bromine-bromine single bond are repelled by the π electrons. This temporary dipole produces an electron deficiency at the end of the molecule nearer the double bond, and thereby allows the electrophilic attack. The intermediate carbocation then reacts with a bromide ion to complete the electrophilic addition.
However, it is possible to intercept the carbocation with other nucleophilic reagents. So, if a chloride ion is also present in the reaction mixture, it can react as a nucleophile; and if the reaction is carried out in the presence of water, a water molecule can react with the carbocation. This means that the choice of solvent used for the bromination is important. If it were aqueous bromine, for example, it would be impossible to stop the interception of the carbocation by a water molecule acting as a nucleophile.
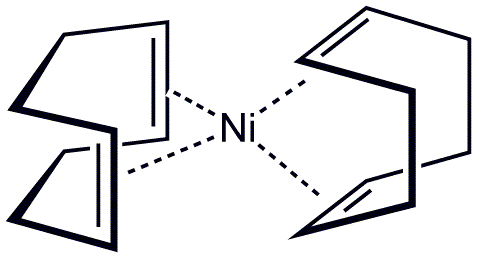
Structure of bis(cyclooctadiene)nickel(0), a metal–alkene complex. Smokefoot, Public Domain
TEST FOR UNSATURATION
Aqueous bromine may be used to test for the presence of a C-C bond, since the reagent changes colour during electrophilic addition. Aqueous bromine is orange, but after reaction with an alkene it forms colourless products. Alternatively, bromine in an inert solvent, such as tetrachloromethane or hexane, can be used. In both of these solvents the same colour change is observed: orange to colourless.
Thanks for reading.
REFERENCES
https://en.wikipedia.org/wiki/Alkene
https://en.wikipedia.org/wiki/Cracking_(chemistry)
https://www.chemguide.co.uk/mechanisms/elim/elimvsubst.html
https://www.chemguide.co.uk/organicprops/haloalkanes/hydroxide.html
https://courses.lumenlearning.com/introchem/chapter/reactions-of-alkenes-and-alkynes/
http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch06/ch6-4.html
https://www.chemguide.co.uk/organicprops/alkenes/hhal.html
https://www.chemguide.co.uk/mechanisms/eladd/unsymhbr.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch15/ch15-2-1.html
http://leah4sci.com/acid-catalyzed-hydration-of-alkenes-reaction-and-mechanism/
https://en.wikipedia.org/wiki/Halogen_addition_reaction
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
hello, really a beautiful article! I have really liked it, I give you the highest grade and I invite you to join the community of stem.curate, you will find in the next comment the information if you want to come and visit us!
a big greeting,
riccc96
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
Please join us on discord.
Thanks, @stem.curate and @riccc96.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.