ALDEHYDES AND KETONES: Sex Hormones and The Pill
The most publicized hormones must be progesterone and testosterone. The reason for their fame is sex. Progesterone is the female sex hormone, which prepares the uterus for pregnancy after an egg has been fertilized, and which prevents the ovaries from releasing any more eggs. Testosterone is the male sex hormone responsible for sexual development and drive in males, and for muscle growth. Although they have very different functions, these hormones are structurally very similar (they are steroids with a carbonyl functional group), and it takes only a simple chemical reaction in the laboratory to convert progesterone into testosterone.
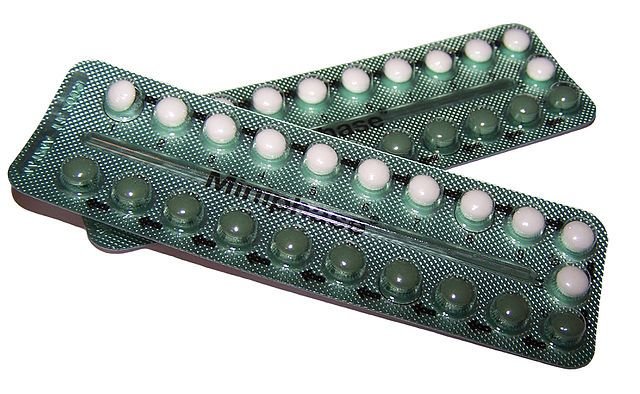
Once their structures became known in the 1940s, chemists set about synthesising them, and soon synthetic sex hormones featured in a number of medical applications. However, few people would have predicted the social revolution that was to follow when synthetic progestins, a group of chemicals that mimic the action of progesterone, were developed. The synthetic progestin norethynodrel was the basis of the first oral contraceptive, known simply as ‘the pill’, introduced in 1960. There is perhaps no more striking example of the influence of chemistry on our lives.
Oral contraceptives are those pills liberated women from the fear of unwanted pregnancies and thereby revolutionized sexual behaviour. However, the occurrence of these chemicals in recycled drinking water have been partly blamed for reducing the sperm count of males.
RECOGNISING AND NAMING ALDEHYDES AND KETONES
Aldehydes and ketones contain perhaps the most important functional group in organic chemistry – the carbonyl group, C=O. Hence, they are known as carbonyl compounds. The carbonyl functional group is found in many important biological molecules, from insect pheromones to human sex hormones. It occurs in the molecules in our eyes that are responsible for vision, and gives lemons their characteristic flavour. It is also involved in the manufacture of many important industrial chemicals, from plastics to solvents.
In aldehydes, the carbon atom of the carbonyl group (the carbonyl carbon) is bonded to at least one hydrogen atom, while in ketones, it is bonded to two carbon atoms, each from either alkyl or an aryl group. Four naturally occurring carbonyl compounds are Menthone which is found in mint leaves; gives a peppermint flavour, Citral: an oily liquid that contributes to the flavour and aroma of oranges and lemons, a moth sex pherome and oestrone: a female sex hormone.
Remember that a functional group is an atom or group of atoms that gives a molecule a characteristic set of properties while a pheromone is a chemical that communicates information between members of the same species and an aryl group contains a benzene ring.

NAMING ALDEHYDES AND KETONES
The systematic naming of aldehydes and ketones is simple. The names of aldehydes end in -al, and those of ketones end in -one. The number of carbon atoms in the chain (including the carbon of the functional group) provides the rest of the name, which is based on the alkane name with the end -e removed. Eight of the more important aldehydes and ketones are methanal, ethanal, propanal, benzaldehyde, propanone, butanone, phenylethanone and pentan-2-one.
METHANAL
Methanal, previously known as formaldehyde, is still used by many industrial chemists. In fact, it is the most-used industrial aldehyde. Methanal is produced by the air oxidation of methanol, using an iron or silver catalyst. Methanal is used to make plastics such as Bakelite (one of the first plastics) and other phenolic resins (for which it is reacted with phenol), urea-formaldehyde resins (for which it is reacted with urea), melamine resins and Formica.
Your kitchen almost certainly contains surfaces and equipment that are products of reactions with methanal – kitchen work tops and pan handles are just two examples. So does your bathroom, because methanal is used as a preservative in some shampoos and bath foams. A 40 per cent aqueous solution of methanal – better known as formalin – is used to preserve biological specimens and as a disinfectant and fungicide.
THE CARBONYL GROUP AND NUCLEOPHILIC ADDITION
As the carbonyl functional group has a double bond, it undergoes addition reactions – just like the double bond of the functional group in alkenes. Alkenes undergo electrophilic addition reactions because the loose electron cloud of the π bond is attractive to electrophiles. There is a π bond in the carbonyl group as well, but it is between two atoms of different electronegativities, C and O. So the density of the electron cloud is greater at the more electronegative oxygen end, thereby making the bond polar.
Since C=O is polar, the electron-deficient carbonyl carbon is susceptible to attack by nucleophiles. A nucleophile has a lone pair of electrons with which it can form a covalent bond. It is attracted to a centre of positive charge. I have already explained the nucleophilic reactions with the polar carbon-halogen bond of halogenoalkanes, and with the polar C-O bond of alcohols. In both these cases, substitution reactions occur. However, the carbonyl group has a double bond, and therefore as the nucleophile attacks and forms a covalent bond with the carbonyl carbon, the π bond splits and forms another single covalent bond, which results in an addition reaction.

Injecting a giant squid specimen with formalin for preservation. Gene Carl Feldman, Public Domain
NUCLEOPHILIC ADDITION BY HYDROGEN CYANIDE
The reaction of hydrogen cyanide (HCN) with the carbonyl group is a very important synthesis reaction, because it adds another carbon atom to the molecule. The reaction with propanone is used in the production of poly(methyl-2-methylpropenoate), better known by its trade name of Perspex. The first reaction in the synthesis of Perspex is the production of a hydroxynitrile (cyanohydrin).
The nucleophilic addition reaction of hydrogen cyanide was one of the reaction mechanisms ever to be investigated: this was by a British chemist, Arthur Lapworth, in 1903. A reaction mechanism shows the steps by which a reaction takes place. The nucleophile is the cyanide ion (CN-), rather than the HCN molecule. HCN is a poor nucleophile, and if CN- ions are not present, the reaction is very slow. Either the addition of potassium cyanide (KCN) can provide the CN- ions or they can be generated from HCN by adding an alkali:
HCN + OH- → H2O + CN-
A proton then bonds to the oxygen atom. Often this proton is transferred from HCN or H2O. The net result is the addition of HCN to the molecule. Notice that in this case the proton was transferred from HCN, and a CN- ion is regenerated. Hydrogen cyanide, HCN, is highly toxic and a concentration of 270 ppm of the gas causes death within ten minutes. KCN is also extremely toxic.
Hydroxynitrile: The Millipede’s Defence
To deter predators, if not finish them off for good, one species of millipede (Apheloria corrigata) uses the deadly gas HCN – hydrogen cyanide – yet manages not to harm itself. This is because inside the millipede are separate stores of a hydroxynitrile derivative of benzaldehyde, which is harmless, and an enzyme that catalyses the compound’s reaction to form benzaldehyde and HCN; the two are not mixed until they leave the animal’s body.
When the millipede is attacked, it discharges both compounds. The enzyme becomes mixed with the hydroxynitrile, and the hapless attacker is enveloped in hydrogen cyanide. Isn’t that interesting!?

Flat-backed Millipede - Apheloria virginiensis corrugata, Jones Preserve, Washington, Virginia. Judy Gallagher, https://www.flickr.com/photos/52450054@N04/26868834455/, CC BY 2.0
NUCLEOPHILIC ADDITION OF H-
The hydride ion (H-) comes from, NaBH4 (sodium tetrahydridoborate(III). The mechanism is similar to that of the addition of hydrogen cyanide, only in this case the nucleophile is H-. Once the NaBH4 has reacted with the carbonyl compound, water is added to provide the protons.
Notice that an alcohol functional group is produced. This reaction is also called a reduction. The equation for the reduction of aldehydes in general is written:
RCHO + 2[H] → RCH2OH
H is put in brackets to signify that it comes from a reducing agent, which in this case is NaBH4 (this is a simplified way to balance the equation and does not represent atomic hydrogen). This reaction will further be discussed again when I will be looking at the reduction of aldehydes and ketones.
LiAIH4 (lithium tetrahydridoaluminate(lll) is a more powerful reducing agent than NABH4. It also reacts with water, which is why it is dissolved in dry ethoxyethane. However, NaBH4 is a more convenient reagent as it can be used in aqueous or alcoholic solution and the reaction is less vigorous.
REACTION WITH 2,4-DINITROPHENYLHYDRAZINE
2, 4-dinitrophenylhydrazine (also called Brady’s reagent) reacts with carbonyl groups to give orange-coloured precipitates, called 2, 4-dinitrophenylhydrazones. This reaction is used to test for the presence of carbonyl group in unknown compounds. The coloured precipitates from the reaction can be purified by recrystallisation to give products that have very precise melting points, which can therefore be used to identify a particular aldehyde or ketone.
The ethanal derivative ethanal 2,4-dinitrophenylhydrazone melts at exactly 168 °C, so its melting point can be used to identify ethanal, a method that used to be important before different types of spectrometers became widely available for identifying substances. Before spectrometers, the best way to recognize unknown carbonyl compounds was to produce from the liquid a solid derivative with a sharp melting point.The melting point is also a good way to check the purity of a sample, since any impurity lowers the melting point.
Addition-elimination mechanism of the 2,4-dinitrophenylhydrazine reaction
The two nucleophilic addition reactions in the previous section produce stable products. The reaction between the carbonyl group and 2,4-dinitrophenylhydrazine gives an unstable product, which spontaneously reacts with the elimination of a water molecule.
The mechanism of the reaction shows that the lone pair of electrons on the first nitrogen atom provides the basis of the nucleophilic addition, and the intermediate compound is then formed by an internal rearrangement. The final product, a 2,4-dinitrophenylhydrazone is formed from the unstable intermediate by the elimination of a water molecule. So this reaction involves both an addition reaction and an elimination reaction – hence addition-elimination.
SUN TAN FROM A BOTTLE
Over-exposure to sunlight is known to cause skin cancer, yet millions of people still work hard to give themselves a suntan because they think it makes them look healthier and more attractive. Despite grim warnings about the consequences, sunbathing still has millions of devotees. However, suntans have not always been fashionable. Until about 200 years ago, upper-class women who went on leisurely walks would protect their pale complexions with bonnets and parasols. This distinguished them from the bronzed working men and women who laboured outdoors in the sunshine.
Attitudes gradually began to change with the industrial revolution. While factory and office workers toiled in buildings that shut out the sunshine all day, better off people began to think of a suntan as a sign of wealth and position in society. This trend grew when it was the fashion in the early 1900s to go on touring holidays of continental Europe – the forerunners of today’s package holidays.
A natural tan is caused by the dark pigment melanin, which is produced in the skin to absorb harmful ultraviolet rays. A similar tan-like effect can be produced after a few hours using DHA, a colourless ketone that reacts with the protein in the outer skin to produce a brown pigment. DHA, traditionally called dihydroxyacetone, is 1,3-dihydroxypropanone:
There are problems with a ‘chemical’ tan. Cells of the outer layer of skin are dead and so within a few weeks these have rubbed off – and so has the tan! Also, because only dead cells react with DHA, where these are in a thicker layer, such as at the elbows and knees, the tan is darker than elsewhere. It is also worth noting that a chemical tan does nothing to protect the skin from harmful ultraviolet rays.

Ball-and-stick model of the dihydroxyacetone molecule. Jynto (talk), CC0
Grignard reagents and aldehydes and ketones
If magnesium turnings are added to a solution of halogenoalkane (RX) in ethoxyethane, an exothermic reaction produces a solution of Grignard reagent:
R–X + Mg → R–Mg–X
R can be either an alkyl or an aryl group and X is a halogen. The reagents are named after their discoverer Victor Grignard, a French chemist. One of the most important uses of Grignard reagents is to synthesise alcohols from aldehydes and ketones. The mechanism for this reaction is nucleophilic addition. The carbon-magnesium bond is highly polar and attacks the carbonyl group to form an intermediate that is decomposed by adding dilute acid.
Primary, secondary and tertiary alcohols can be made using this reaction.
Thanks for reading.
REFERENCES
https://www.sexandu.ca/contraception/hormonal-contraception/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2852204/
https://medlineplus.gov/druginfo/meds/a601050.html
https://slideplayer.com/slide/8330926/
https://www.angelo.edu/faculty/kboudrea/index_2353/Chapter_04_6SPP.pdf
http://www.docbrown.info/page06/AldehydesKetones.htm
https://www.essentialchemicalindustry.org/chemicals/methanal.html
https://en.wikipedia.org/wiki/Formaldehyde
https://courses.lumenlearning.com/suny-potsdam-organicchemistry2/chapter/20-5-addition-of-hcn-to-co/
http://www.docbrown.info/page06/OrgMechs3b.htm
http://shout.education/ChemKey/mechanisms/nucadd/hcn.html
https://www.chemguide.co.uk/mechanisms/nucadd/hcntt.html
https://www.mdpi.com/2075-4450/9/2/51/htm
https://www.jstor.org/stable/26464006
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.