ENERGY AND TEMPERATURE
Like most of my articles, this article was written only after a lot of late nights and numerous cups of coffee – usually ‘instant’ coffee! The instant coffee industry has developed into a multi-million-pound market since it first began in the late 1930s. And one of the latest development “freeze-dried’ granules, which give the coffee a much better flavour than all the earlier versions.
Once the coffee beans have been roasted and ground, the soluble solid and flavour compounds are extracted. This mixture contains a lot of water, which would make granules stick together. The problem is to remove the water without removing the flavour.
In freeze-drying, the mixture is cooled to just below 0°C (it is still sticky and air is added to make a ‘foam’ that is of the right density. This foam is then deep-frozen and broken into the familiar airy granules we see in the coffee jar. The granules are then heated very rapidly in a drying cabinet at very low pressure so that the ice converts immediately to vapour without passing through the liquid stage, leaving behind almost all of the aroma and flavour of the original coffee.
The largest modern freeze-drying plants can produce up to 7 million cups of coffee each day – more than enough for several more physics articles! Lol.
The ideas in this chapter……..
When we switch on an electric fire in the home, a current passes through the element – usually a coiled wire. The electrical energy for this is provided at the power station by the chemical change when gas, oil or coal is burned. This energy is then transferred via the resistive coil of the fire to air molecules in the room. The air molecules in the room start to move around faster in every direction. This increased movement constitutes the so-called ‘heating’ of the air.
We can transfer energy quite efficiently from the fire to the air molecules. But we cannot easily reuse the energy and change back this increased movement of the air molecules to energy that can be returned along our electrical circuit. There are certain restrictions on the transfer of energy. Thermodynamics is the science that describes how systems alter when their energy content changes. It describes and explains the transfer processes.
We can define heating as a process in which energy transfers from one body at a higher temperature to another body at a lower temperature. We shall look at practical methods of measuring temperature in this chapter. Different forms of energy are so important in physics that they are discussed and explained. When we heat a substance we transfer energy from some outside source (as I have indicated) and this energy goes to the atoms or molecules of that substance. The kinetic energy of these atoms or molecules increases. So, often, does the separation of the atoms or molecules. If the separation changes, so does the force between them. Essentially, then, this extra energy given by heating is an increase in both the kinetic energy and the potential energy of the components. For this reason, many scientists do not like to use the term “heat”, which would appear to describe a unique form of energy.
The energy of the atoms or molecules inside the substance (the internal energy) can be increased not only by flow of energy but also by doing work. It is therefore usual in advanced physics to refer to these two contributions as heat and work. It is not until the next article that we shall look at the first law of thermodynamics, which states how these contributions change the internal energy and hence why a so-called form of energy called heat is in fact often used. The energy flow referred to as heat (or thermal) flow is a random process, whereas when we do work we exchange energy in some ordered manner. Order and disorder are also discussed in more detail later on.
ENERGY AND WORK
Historically, the nature of heating greatly puzzled scientists. This is because they did not know then what we now understand about the structure of matter – that it is made up of atoms. Count Rumford in the late eighteenth century became interested in the way thick metal rods became hot when bored out to make cannons. But a great step forward came when Joule showed that mechanical work could be transferred to thermal energy, which resulted in an increase in temperature of water in a cylinder.
We can demonstrate the transfer of mechanical work to thermal energy (as internal energy) using lead shot in a closed cylindrical tube. The tube is repeatedly inverted so that the lead shot falls from end to end each time. When the lead shot weighs 150 g (0.15 kg) and each fall is 0.75 m, we can calculate the rise in temperature of the lead shot after 100 falls.
.As the masses fall, work is done as a result of the gravitational force. This work is given by:
Work done = mass of lead × Acceleration due to gravity × length of fall × number of falls
= 0.15 × 9.8 × 0.75 × 100 J = 110 J
- The work is transferred to kinetic energy as the lead shot is falling.
- But the lead shot is brought to a sudden halt at the bottom of the tube. Now the energy transfers to internal energy within the shot. As a result, the amount of movement and the potential energy of the lead atoms are increased. Overall, the work done has been used to raise the temperature of the lead by ΔT.
- The specific heat capacity for lead is 129 J kg-1 K-1. The amount of energy given to the lead shot is given by:
Energy (in J) = mass of lead × Specific heat capacity of lead × temperature rise
= 0.15 × 129 × ΔT = 19.4 x ΔT
- Equating work and energy (in J):
110 = 19.4 × ΔT
So the temperature rise is:
ΔT = 110/19.4 = 5.7 K
This temperature rise will be detectable even when there are some energy losses to the surroundings. Note that the mass of the lead shot is used twice, once for the work done and once for the energy transferred to the lead shot. This means that the temperature rise does not depend on the mass of the shot used and need not have been given in the overall calculation. Increasing the mass of shot increases the amount of required work but also increases the mass to be heated. In the calculation, we could use a symbol for mass and then cancel mass on either side of the equation.
Joule’s work on thermodynamics
James Prescott Joule came from a family of wealthy brewers in Salford and conducted his early experiments in a laboratory at the brewery. He was wealthy enough to fund his own experiments. He devised an accurate thermometer and on his honeymoon he spent time measuring the temperature difference between the top and bottom of a scenic waterfall.
Joule’s first thermometers measured to within ±0.02 °C and later ones to ±0.005 °C. It was a great achievement to measure temperatures so accurately, and necessary for Joule’s study of the conversion of mechanical work to energy.
Joule had been educated privately at home, with no formal education in mathematics, so that he could not on his own keep up with the new science of thermodynamics. However, he won the support of William Thomson (Lord Kelvin) and together they made great advances in thermodynamics, Joule providing the practical ability and Thomson the theory.
THERMAL TRANSFER OF ENERGY
There are three types of thermal transfer of energy: conduction, convection and radiation.
CONDUCTION
Energy is transferred within solids by the process called thermal conduction. The atoms in a solid vibrate, and as the temperature increases, the amplitude of vibration increases. The energy of the atoms increases and the internal energy of the solid is increased. The vibration of the atoms is rather like that of a set of masses connected to a lattice of springs.
Energy travels through a solid by way of vibrations through the system. Neighbouring atoms are set into increased vibration one after another. In metals, ‘free’ electrons travelling among the atoms also gain extra energy and travel a little faster as the temperature increases. Importantly, they are the means of energy transport through the metal and they pass on energy to more distant atoms.
CONVECTION
There is movement of internal energy in a fluid (a liquid or a gas) by process of thermal convection (conduction also occurs). The fluid particles, usually molecules, are free to move around independently. The kinetic energy increases as more thermal energy is added to the fluid. Heating the fluid causes it to expand and become less dense. Because of the effect of gravity, the less dense (higher temperature) fluid rises up through the more dense fluid, which then falls to take its place. This movement is a very effective way of transferring those molecules that have been given extra kinetic energy.
Currents of molecules are set up called convection currents. Convection currents cause thermal energy to circulate in kitchen ovens. Cooks know that less dense hotter air rises to the top of the oven. When cooking more than one item of food, they choose the appropriate shelf for each item. Many modern ovens include an electric fan, which both increases the circulation rate and produces a more uniform temperature distribution. This is called forced convection, whereas if the thermal energy is left to distribute itself, the process is called natural convection.
RADIATION
Energy can be transferred in the form of electromagnetic radiation. All objects emit and reflect electromagnetic radiation. We are aware of the radiation in the form of light waves. However, as the temperature of an object increases, both the range of frequencies and the quantity of radiation given out increase.
An electric fire radiates much of its energy in the visible region – we see the element glowing orange or red. Stars are much hotter and radiate much of their energy in the ultraviolet region.
On the other hand, thermal imaging cameras can pick up infrared radiation at longer wavelengths and smaller frequencies than the visible range. This means that hot bodies can be detected against a cooler background. Imaging cameras are used by the fire service to detect people overcome by smoke in buildings on fire. When slung from police helicopters, these cameras can detect suspected criminals on the run at night.
Black body
An object that completely absorbs thermal radiation at all wavelengths is called a black body. It reflects none of the radiation falling on it, yet it can emit radiation at all wavelengths. The figure below shows the radiation curves for a black body.
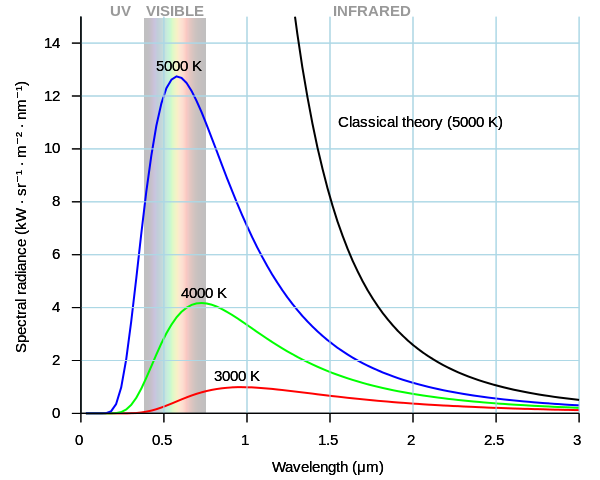
Radiation curves for a black body. Darth Kule, Public Domain
A furnace with a very small opening and thick, insulating walls approximates to a black body. Radiation directed into the furnace through the opening is absorbed by the inside walls. However, the furnace walls will be very hot (they will be at the temperature of the furnace) and so will be emitting radiation at all wavelengths, a small amount of which will escape through the opening.
As the temperature increases, the amount of radiation from the black body also increases (corresponding to the area under the curve). The wavelength at which peak emission occurs falls as the temperature increases. This is why when a piece of metal such as steel is heated, it first appears to be a dull red but, as it gets hotter and the peak in the emitted light moves to smaller wavelengths, its appearance becomes more orange.
MEASURING TEMPERATURE
The properties of an ideal gas can be used to define a temperature scale that is reproducible anywhere. This scale is the Kelvin scale. Also, a constant-volume gas thermometer measures temperature. But such a thermometer is not easy to carry around and to use. Temperature can be measured using any physical property that varies with temperature in a reproducible way. But we must be able to calibrate the variation against our standard Kelvin scale as obtained with the gas thermometer. We do not have to stay with the Kelvin scale itself, but the only other scale now in common use is the Celsius scale.
Examples of physical properties that we might use are:
- length, such as the length of a column of alcohol in a liquid-in-glass thermometer,
- voltage, such as that produced with a thermocouple,
- electrical resistance of a wire (often platinum) or semiconductor (thermistor),
- pressure, as in a gas thermometer,
- thermal radiation (using a pyrometer).
TEMPERATURE SCALES
The thermodynamic scale (or absolute scale or Kelvin scale): In this type of scale, a single fixed point is taken at the triple point of water and defined as 273.16 K. The unit of temperature, the kelvin, is 1/273.16 of this triple point temperature. The size of the unit then determines at what stage we get down to the zero of the scale. There is no negative temperature and we can never quite reach zero in the laboratory.
Because scientists need to make measurements over a very wide range of temperatures, they have defined some additional fixed points for calibrating thermometers. However, only the triple point is fundamental to all scales.
The Celsius scale
The Celsius scale is derived from the thermodynamic (Kelvin) scale using the same single fixed point as the thermodynamic scale. But, in Celsius, the ice point is 0.01 °C (usually assumed 0 °C for practical purposes) and the steam point is 100.00 °C.
The relationship is:
t = T – 273.15
Where t is the temperature on the Celsius scale and T is the temperature on the thermodynamic scale.
Physical properties to calibrate temperature
Calibration is easy if we are measuring a change of length. More generally, though, we need to know the value of the property (here, length) at the fixed points and calibrate using a proportionality.
Let us see this in action for a mercury-in-glass thermometer:
- l100 is the length of mercury thread at 100°C.
- l0 is the length of mercury thread at 0 °C.
- lθ is the length of mercury thread at θ °C.
- Each degree interval will have a length (l100 - l0 )/100.
- The change of length due to a temperature rise from 0°C to θ °C is (lθ - l0).
- The temperature θ in degrees is given by change of length divided by length per degree interval:
θ = [lθ - l0/(l100 - l0 )/100](degrees) = (lθ - l0/ (l100 - l0 ) × 100°
We could also use a platinum resistance thermometer to measure the resistances R, at 0°C, R100 at 100°C and Rθ at an unknown temperature θ °C:
θ = (Rθ - R0/ (R100 - R0 ) × 100°
All mercury-in-glass thermometers should agree precisely if they are calibrated correctly. So should platinum resistance thermometers. Also, all thermometers of either type should also agree with each other. However, we cannot assume that, for every degree change in temperature, the length of the mercury thread or the resistance of the platinum changes by exactly the same value. For example, the changes in length of the mercury column between 9°C and 10°C and between 40°C and 41°C may differ. Similarly, resistances at different degree intervals may vary. So the two types of thermometer may not give precisely the same temperature readings between fixed points. Hence our need for the absolute scale.
Mercury-in-glass thermometers are not often used in modern laboratories in case of breakage. Mercury has a significant vapour pressure and is slightly toxic.
USE OF THERMOMETERS
The choice of a thermometer will depend on the temperature range and where it is used. Mercury thermometers are no use below -39°C, which is the freezing point of mercury. But they can be used over a wider range of temperature than the alcohol-in-glass thermometer. A large thermometer is not suitable for measuring the temperature of small amounts of material. This is because, while the thermometer achieves thermal equilibrium, energy could be transferred to or from the material and so the temperature being measured could change. A resistance thermometer is a likely choice if high accuracy is required.
REFERENCES
https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/a/heat
http://www.physicalgeography.net/fundamentals/6c.html
https://www.khanacademy.org/science/physics/work-and-energy
https://www.khanacademy.org/science/physics/work-and-energy/work-and-energy-tutorial/a/what-is-work
https://en.wikipedia.org/wiki/Work_(physics)
https://en.wikibooks.org/wiki/Engineering_Thermodynamics/First_Law
https://schoolworkhelper.net/thermal-energy-transfer-conduction-convection-radiation/
https://en.wikipedia.org/wiki/Radiation
https://en.wikipedia.org/wiki/Black-body_radiation
https://en.wikipedia.org/wiki/Black_body
https://en.wikipedia.org/wiki/Temperature
https://en.wikipedia.org/wiki/Temperature_measurement
https://www.toppr.com/guides/science/heat/heat-and-measuring-temperature/
http://abyss.uoregon.edu/~js/glossary/temperature_scale.html
https://www.britannica.com/technology/Celsius-temperature-scale

This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.