ATOMIC NUCLEUS: Forces In The Nucleus
THE ELECTRICAL FORCE:
The positively charged protons exert an electrical force of repulsion on each other.
The force between two protons, of equal charge +e, can be calculated from the
formula:
FE = ke2/r2
where k is the electric force constant (9 × 109 N m2 C-2) and r is a typical nuclear distance, about 10-14 m. So the force between the two protons in a helium nucleus is 2.3 N. This is a repulsive force and is immense on the nuclear scale because protons are so close together. The force would, on its own, cause the protons to fly apart at high speeds in a nuclear disintegration. But this doesn’t happen!

Image by Pete Linforth from Pixabay
THE STRONG NUCLEAR FORCE
Nuclei do exist and are quite hard to break apart. So there must be another force, stronger than this electrical repulsion force, to attract nucleons to each other and so keep the nucleus together. This is the strong nuclear force, about 100 times as strong as the electrical force in the nucleus. Unlike electrical and gravitational forces, the strong nuclear force has a very short range: it reaches only as far as from one nucleon to its next neighbours. This force affects both neutrons and protons. However, large nuclei have a problem: there comes a size when the long-range electrical force from a large number of protons will overcome the short-range nuclear force. Two things may then happen:
- A stable ‘unit’ of matter might escape – the unit is an alpha particle. Larger nuclei tend to be radioactive alpha emitters (e.g. Th-232, Th-228, Ra-224, Rn-220, etc.).
- The nucleus simply breaks apart into two roughly equal smaller nuclei – this is nuclear fission, which happens with uranium and is the main source of nuclear power.
CHEMISTRY AND ATOMIC STRUCTURE
In relating the chemical behaviour of an element to its place in the Periodic Table. More and more nucleons can join together to make larger and larger nuclei, and the patterns will emerge linking atomic structure and chemical behaviour. In chemical reactions, the outer electrons of atoms interact. Elements with the same number of outer electrons react similarly, and are arranged in columns in the Periodic Table called groups. Examples of groups include the alkali metals (lithium, sodium, etc.), the halogens (fluorine, chlorine, etc.), and the noble gases (helium, neon, etc.).
ISOTOPES
All nuclei except the hydrogen nucleus contain both protons and neutrons. For the smaller nuclei, the number of neutrons is about equal to the number of protons – but this is a very rough rule. All atoms of an element have the same number of protons, but they may have different numbers of neutrons and are then called isotopes of that element. The mass of an atom of one isotope of an element will then be different from the mass of another isotope. For example, the nucleus in the most common atom of carbon has 6 protons and 6 neutrons. But we can find seven forms of carbon nuclei with different masses. So carbon has seven isotopes. They all have the same number of protons (and electrons), so they occupy the same place in the Periodic Table and have identical chemical properties.
Only carbon-12 and carbon-13 nuclei are stable; the rest are unstable due to an imbalance of protons and neutrons and are radioactive. A nuclide is the name of a particular isotope and is shown by a ‘formula’ giving both its mass number and its proton number: 22888Ra. The formula tells us that this nuclide is an isotope of radium (Ra) with a mass number (protons + neutrons) of 228 nucleons, and a proton (or atomic) number of 88. Thus there are 88 protons and 140 neutrons in the nucleus, and the atom would be surrounded with a cloud of 88 electrons.
Relative atomic mass
The mass of an atom is very small. So it is usually given as a multiple of a standard unit, the unified atomic mass unit, u. This multiple is called the relative atomic mass or RAM. This is usually quoted as the mean of the relative atomic masses of the naturally occurring isotopes. The atomic mass unit is defined as one-twelfth of the atomic mass of the commonest isotope of carbon (carbon-12).
MEASURING NUCLEAR MASSES USING THE MASS SPECTROGRAPH
Accurate measurements of the masses of nuclei are made using the mass spectrograph, as is in the figure below.
This device vaporises atoms and then ionises them with a beam of electrons. The ions are accelerated and enter the spectrograph chamber which separates ions of different mass into different paths. The separation is done using a magnetic field directed out of the plane of the diagram. The magnetic field exerts a force at right angles to the direction of motion of the ions – so providing a centripetal force to make the ions move in a circular path. A centripetal force F makes a mass m move in a circle of radius r at a speed v such that:
F = mv2/r
F is a magnetic force of size Qv × B for a field of flux density B acting on a charge Q travelling with speed v, so:
F= QvB = mv2/r
and so the radius of the path is given by:
r = mv/BQ [1]
This simple theory assumes that all the atoms are ionised to the same degree, so that the charge Q is the same for them all, namely e, the electronic charge.
The velocity selector
Note that equation 1 involves the speed v. If we want all ions of the same mass and charge to follow the same path, they must also have the same speed. This is achieved by the velocity selector in the figure. Ions enter the selector with a wide range of speeds and are in both a magnetic field BS and an electric field ES. These fields are at right angles to each other and to the direction of the ion beam, with the result that the electric force (QES) and the magnetic force (QvBS) on the ions act in opposite directions. When these forces are equal, the ions are undeflected and carry on to go through the exit aperture into the deflection chamber. But the forces only balance out at a particular value of v, when:
QvBS = QES
so that:
v = Es/Bs [2]
Ions with different speeds are deflected out of the straight path, so do not pass into chamber. The strength of the deflecting field B in the chamber is varied, so bringing, in turn, ions of different mass into the detector. The detector counts individual ions and so measures the relative quantities (abundances) of ions of different mass in the sample. By combining formulae 1 and 2 above:
r = mv/BQ and v = Es/Bs
we get: mES/BQBS and so: m = QrBBS/ES
This boils down to: m = kB [3]
where k is a constant.

The unified atomic mass constant, u
It is hard to measure the constant k in equation 3 accurately, so values of m for different ions are measured by comparison with a standard nuclear mass. This is the nucleus of carbon-12, which is defined to have a mass of exactly 12 unified atomic mass constants (12 u), where u is 1.660 540 2 × 10-27 kg. In energy terms this is equivalent to 930 MeV. On this scale, the mass of a proton is 1.007 276 5 u.
THE SIZE OF A NUCLEUS: A Simple Theory
Protons and neutrons have almost exactly the same mass. Assuming that they also occupy the same space, volume v, the volume of a nucleus with A nucleons is Av. If the nucleus is spherical with radius r, then we can write:
approximate volume of a nucleus = Av = 4πr3/3
This result is usually written as: r = r0A1/3
where r0 is an experimental constant of value 1.2 × 10-15 m.
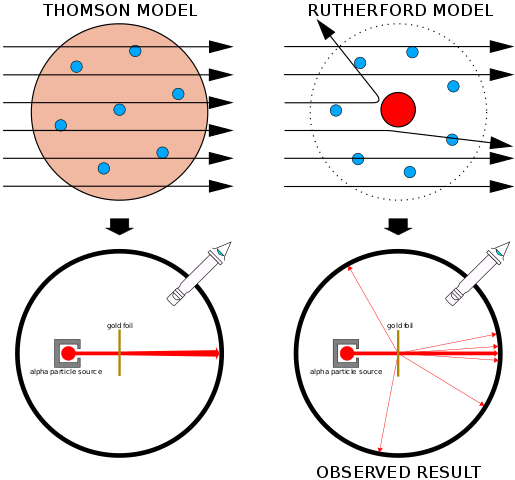
THE EXPERIMENTAL EVIDENCE
When a metal is bombarded with a beam of charged particles, some of the particles bounce back almost exactly along the approach path. The first such experiment used alpha particles and led to the discovery of the nucleus.
What happens is shown in the figure below. The approaching particle is repelled by the nucleus. Its kinetic energy EK is converted to electrical potential energy Ep. The point of closest approach occurs when all the EK becomes Ep, that is:
½ mv2 = kqQ/d = k(2Ze2)/d
Where k is the electric force constant (which is 9 × 109 Nm2 C-2) and the other quantities are as shown in the figure. The energy of the approaching particle is known and so are the charges of the particle and the nucleus. This means that we can calculate d. A missile with high energy gets very close to the nucleus, so d is very nearly the same as the nuclear radius r. Experiments similar to this in which electrons are scattered by nuclei have shown that most nuclei are approximately spherical with a size given by the relationship r = r0A1/3 with the value of the constant r0 as given above.
NUCLEAR DENSITY
The volume of a nucleus is V = 4πr3/3 where r = r0A1/3. Inserting this value gives:
V = 4πr03A
For iron A = 56. Check that the volume of an iron nucleus is 1.22 × 10-42 m3. Each nucleon has a mass of 1 u or 1.67 × 10-27 kg, so the iron nucleus has a mass of 9.35 × 10-26 kg.
Thus the (approximate) density of an iron nucleus is
dn = mass/volume = 7.7 × 1016 kg m-3
This is a huge density: a matchboxful of nuclear matter would have a mass of 5 billion tonnes!
In my next post, I will discuss how nuclei change as a result of radioactive decay. Till then, I remain my humble self, @emperorhassy.
REFERENCES
https://study.com/academy/lesson/electric-force-definition-equation.html
https://en.wikipedia.org/wiki/Nuclear_force
http://aether.lbl.gov/elements/stellar/strong/strong.html
https://en.wikipedia.org/wiki/Strong_interaction
http://www.chem4kids.com/files/atom_isotopes.html
https://en.wikipedia.org/wiki/Isotope
https://www.sciencedirect.com/topics/engineering/relative-atomic-mass
https://en.wikipedia.org/wiki/Relative_atomic_mass
https://www.jstor.org/stable/43420170
https://www.britannica.com/science/mass-spectrograph
https://en.wikipedia.org/wiki/Dalton_(unit)
http://www.theochem.ru.nl/~pwormer/Knowino/knowino.org/wiki/Unified_atomic_mass_unit.html
https://www.britannica.com/science/Rutherford-model
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.