Alkyl polyglycoside
Alkyl polyglucosides
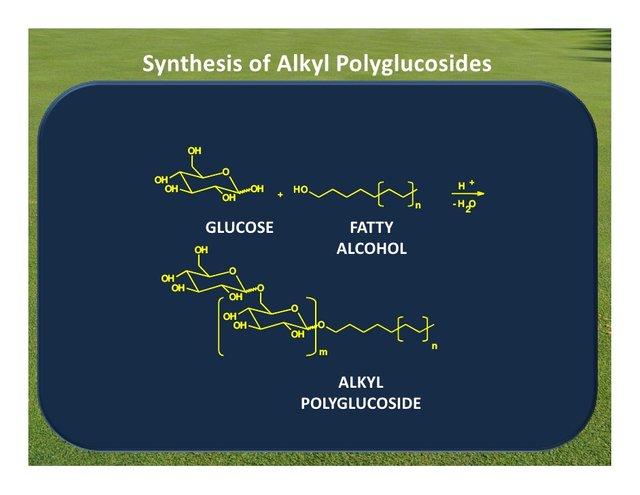
source
The alkyl poly glucosides (APG) are non-ionic surfactants towards which The scientific community has recently turned a blind eye, due to the fact that, in addition, to possess interesting interfacial properties (Nickel, 1992), exhibit excellent ecological and toxicological properties (Andree, 1991). These compounds began to use as ingredients for detergents and cosmetic products (Balzer, 1996) and will have a great future if the research achieves, by studying the synergisms that appear mix them together, optimize their physical-chemical properties.
Emil Fischer synthesized and identified the first alkyl glycoside in 1893, but these products have only had academic interest and were not used commercially until in the 40-50s, several companies developed the business processes for it is manufactured. It was in 1992 when Henkel inaugurated the first plant in the USA. with a production of 25,000 tons per year and in 1995 the second plant with equal capacity per Henkel KgaA in Germany.
Regarding the industrial synthesis of these surfactants, it is worth mentioning that developed enormously in recent decades by improving the process of glucosidation, Fischer (1893); process based on the acid catalysis of glucose with alcohols. Thus, for example, in 1997 a total world production of 8 · 107 was already reached kg (Eichhorn, 1999).
The raw materials for the manufacture of alkyl polyglycosides are alcohols fatty and different sources of carbohydrates. Fatty alcohols can be obtained from petrochemical sources and renewable natural sources such as fats and oils; these fatty alcohols, which give the alkyl glycoside the hydrophobic part of the molecule, they are obtained after the transesterification and fractionation of fats and oils, and subsequent hydrogenation of the corresponding methyl esters of fatty acids. Depending on the type of oil or starting fat the composition of the alkyl polyglucoside is different.
The hydrophilic part of the APG is provided by the corresponding carbohydrate. At the production process can be used polymeric carbohydrates or monomers as Raw materials, ie glucose, starch, glucose syrups with low or high content in equivalent dextrose, etc.
Formally the APG are described in terms of an acetal structure, R represents the alkyl group of 8 to 16 carbon atoms, DP is the degree of polymerization (average number of glucose units per alkyl radical), is always greater than 1 and usually less than 2. APG has a typical degree of polymerization from 1.3 to 1.7.
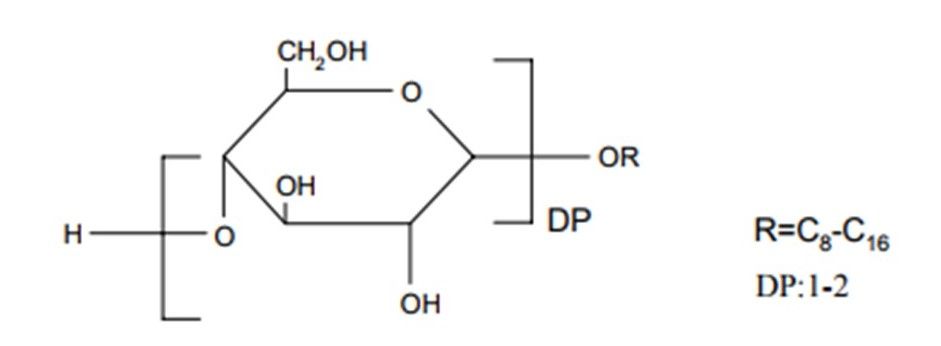
Structure of Alkyl polyglycosides
In the commercial APG, there are more or less complex mixtures of compounds of the type described above. These structures can be differentiated in the number of glucose units present but also in the length of their alkyl chains and even in the possible isomeric differences of the cyclic groups (configuration α or β). That is, commercial APG are complex mixtures of homologs and isomers.
The addition of APG to the detergent formulations improves the stability of the enzymes (proteases, amylases, lipases, and cellulases) during the storage period of the product (Hill, 1997).
In traditional wastewater treatment systems, APG present 100% biodegradation, in methanogenic conditions the alkyl polyglycosides linearities are mineralized by 70%, although branched APG present a certain resistance to degradation (Madsen, 1996).
Among the innumerable advantages presented by these interesting surfactants the following stand out:
• They are based on raw materials renewable.
• They have excellent properties as a surfactant: properties interfacial (Balzer, 1996) and phase behavior (Platz, 1994).
• Its foaming power is better than for other nonionic surfactants (Steber, 1995).
• They are compatible with all types of substances.
• They support all pH range.
• They are applicable in textile detergency, of surfaces and cosmetics.
• They have an excellent compatibility with the skin and mucous membranes (Salka, 1993).
• They are the most biodegradable of all known.
• The critical micelle concentration is very low so it is needed a minor active material, which also It degrades very quickly.
• The metabolites of biodegradation are only CO2 and water, no metabolites are formed recalcitrant during the elimination biological (Steber, 1995).
• They present several types of synergism with some anionic surfactants (Fornara, 2000).
For more information visit the following links.
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/alkyl-polyglycoside
https://www.elsevier.com/books/alkyl-polyglucosides/pantelic/978-1-907568-65-7
How interesting your post. Very good information. :)
Congratulations @ejrangel! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP