Law of Lavoisier // Analysis
As regards the atomic theory, the existence of the molecules does not alter too much our vision of the hierarchic organization, of the nature from the equal atoms and the different types get together in a few portions defined and cause the molecules of the compounds. The chemical reactions are explained on having considered the reorganization, rupture or formation of molecules, the combination of atoms corresponding to two more different elements.

Antoine Lavoisier (1743-1794 ), source of image of mastery of Wikimedia Commons
Law of Lavoisier
In any chemical reaction the mass of the reagents is equal to that of the products dela reaction, this law, if we observe her from the current point of view, it is very logical it was not discovered up to the last quarter of the XVIIIth century, due to the difficulties of measurement, which existed in that epoch, principally in the measurement of mass of the gases, inside it was considered this philosophy that a mass of iron in the outdoors the observer saw, that to average that was spending the time the iron was oxidizing, of such form it was turning into lime, according to the language of that century and his mass it was growing while the oxidation lasted. If we reproduce the same process inside a closed receptacle, the mass will remain invariable, it is easy to understand, that if we weigh the counterfoil, which Zn contains in granalla together with the funnel, which contains hydrochloric acid before the reaction and we repeat the operation later, the mass of the hydrogen, which has taken place will provoke a decrease of the mass of the set, but the serious mass the same if the test with the receptacle closed hermetically.
This law was confirmed across the experiments carried out by Antoine-Laurent de Lavoisier, although it had been used as hypothesis, for other chemists previous to him. The law of conservation of the mass is not rigorously exact it to realize well.
Nowadays it is known, that the mass and the energy are mutually interconvertible in accordance with the equation of Eintein, E = mc2, where E is the energy involved in the trasformación, m is the mass and c is the speed of the light, that you cost 300.000 Km/s. in the chemical reactions habitually the masses of the reagents diminish between 10 ^-9 and 10 ^-11g, quantities that turn out to be completely invaluable.
A philosopher might notice that, even after having hired the beginning the beginning of the conservation of the mass, in a pair of million reactions, one can find concrete reaction, which false completely the beginning: in substances, the empirical hypothesis and therefore, conclusive not reason in against, that earns or weight loses in the course delas chemical reactions. This objection will be valid if the hypothesis of Lavoiser, it had stayed in the state of free hypothesis, but it has stopped having importance in the moment itself in, that the hypothesis has been theorized.
The scientific investigation: his strategy and his philosophy - Page 684 for Mario Bunge – 2000.
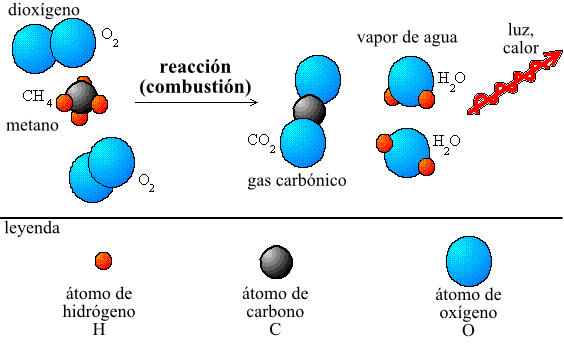
Also it is seen from another point, since in a chemical reaction the atoms do not disappear, simply they are ordained otherwise, Lavoisier had to realize numerous and meticulous experiments to convince those who for then were thinking that, on having warmed a metal this, it was gaining mass when it was turning into a new substance. Lavoisier measured in a closed receptacle the masses of the solid one and the air earlier and after the combustion and came to the conclusion that to mass that was gaining the metal it was equal to the air mass that was getting lost.
Some of the example apart from I shoe, it was that of the combustion of a sail, when it burns it neither is gained mass nor gets lost. The entire mass of the wax and of the molecular oxygen (O2) present before the combustion is equal to the entire mass of carbon dioxide (CO2), water steam (H2O) and wax without burning that they stay when the sail goes out.
Mass of wax mass of O2 = Mass of CO2 Mass of H2O Mass of wax without being burning hot.
Is necessary to admit, that this law for this scientist, you offer to us big contribution in today in the modern chemistry, also they are applied as base of studies of photosynthesis, the combustion, the law of conservation of the mass, the caloric theory, the animal respiration, the oxidation of a fruit.
Bibliography
The scientific investigation: his strategy and his philosophy - Page 684 for Mario Bunge – 2000.
The law of Lavoisier: essays (and errors) 1998-2007 for Nicolás Alvarado – 2007.
basic Operations of the process, miscellanies and dissolutions. QUIE0108 for Sergio Hurtado Melo – 2014.
Congratulations @dark69! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board of Honor
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard: