What did Becquerel and the Curie´s do to win the Nobel Prize for physics in 1903?
Welcome back science-loving friends to a new installment of my series of Nobel Prize winners in physics, where I share a highly digestible and equation-free summary of the work that revolutionized science and for which these very famous scientists were awarded. In this opportunity I have to talk about the 1903 Nobel Prize that was shared by 3 people "Antoine Henri Becquerel, Maria Skłodowska-Curie and Pierre Curie", first for their work in discovering "Spontaneous radioactivity" and then the Curie family for complementing Becquerel's work on "Radiation phenomena".
This work is closely related to Röntgen's discovery of "X-rays", he mentioned many particular phenomena such as radiation, radioactivity, radiations. All this was very fascinating and at the same time disturbing because he could only discover this phenomenon but at the same time left many questions.
Röntgen's work was impressive and describes X-rays in a wonderful way, many in his time thought he was crazy, that he did not know what he was talking about, however after his discovery and giving a true explanation of this phenomenon, many scientists decided to check these results by performing different experiments that corroborated what Röntgen had said. In reality these scientists did nothing innovative, they simply replicated the work of the German with different materials of the time, but it did not explain where these mysterious X-rays came from, which gave rise to various questions that I will now clarify little by little in a very simple way.

CC BY 3.0
When the Frenchman Antoine Henri Becquerel" read Röntgen's work he was fascinated by his discovery and the data provided by the German and decided to put all his efforts into explaining where these mysterious X-rays came from.
If we remember a little of my first installment, I mentioned that to produce the famous X-rays Röntgen used the cathode ray tube, which at the same time produced a very particular photoluminescent phenomenon that was reflected on a screen with a substance. It was here that the Frenchman asked himself the question: "if such a photoluminescent glow is associated with the radiation discovered by Röntgen, does this mean that anything that shines must be completely associated with X-rays?.
At that time there were several salts that contained photoluminescent or phosphorescent properties, so detecting radiation was not complicated. Becquerel knew beforehand that not every shiny object was associated with the X-rays discovered by Röntgen. So the answer to Becquerel's question is that many photoluminescent phenomena have nothing to do with X-rays, something that was perhaps a bit disappointing if he managed to discover that this only happened by simple chance. The idea was to be able to explain where this particular phenomenon came from in some objects, and that is where he carried out his famous experiment below to explain this phenomenon:
Becquerel took uranium salt and placed it in the sunlight for a certain time, then took the salt and rolled it up in a metallic paper and placed photographic plates around it with the idea that they could detect the radiation emitted by the salt. After some time he came closer and saw that the photographic plates were impregnated with a kind of radiation. What did this mean, could it be that the salt was absorbing the solar radiation as well as most of the photo-luminescent substances and then emitting the energy that they had absorbed? Becquerel, to get this doubt out of his head, carried out another experiment where he used the uranium salt in the same way, but this time he did not expose it to the sun, and in the same way the photographic plates were veiled. To check what was happening he placed a variety of substances between the uranium salt and the plates, one of these was the copper sheet in the shape of a cross and he could observe the following:
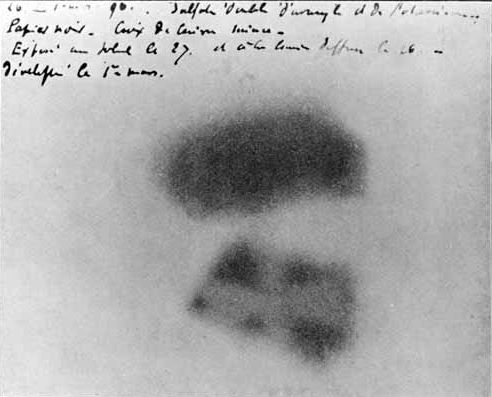
The form of the uranium salt is clearly visible together with the shadow of the copper cross. CC BY 3.0
After observing the result of his experiment he was able to see that the uranium salt emitted a very particular kind of "spontaneous radiation" that belongs to the material and not because it was exposed to solar radiation. Becquerel stated that some kind of radiation is passing through various substances and they were able to produce a photographic film. But what was this, the X-rays discovered by Röntgen or was it another phenomenon similar to this, but if not the X-rays then what were they and what properties does this type of radiation have? Becquerel remained uneasy and wondered where the uranium salt was getting its energy from in order to emit this radiation. He continued to carry out experiments in different ways to be able to check and answer this question and the result remained the same, the salt emitted this radiation in a very strange way all the time, being exposed to the sun or not, leaving it in dark, warm places, waiting one or several hours, days, weeks and the result did not change. Its energy came out of nowhere without any explanation and it didn't run out at all. Therefore, after several experiments Becquerel concludes the following:
Subsequent experiments have shown that uranium activity does not decrease significantly over time.
Becquerel concluded that it was "uranium" that caused this radiation. Although later it was the Curie who reached this final conclusion after checking the work of Becquerel after clarifying all the doubts that the French physicist had left in the air.
But what did Marie and Pierre Curie do?
As I mentioned they continued Becquerel's work. Marie" dedicated herself specifically to studying the radiation emitted by Becquerel's uranium salts and others with which the Frenchman had not experimented. This is how it all began, in principle they had no idea what this radiation was, they simply knew that the source was uranium and since it had no particular name, Maria called this radiation "Becquerel's rays" analogous to Röntgen's X-rays.
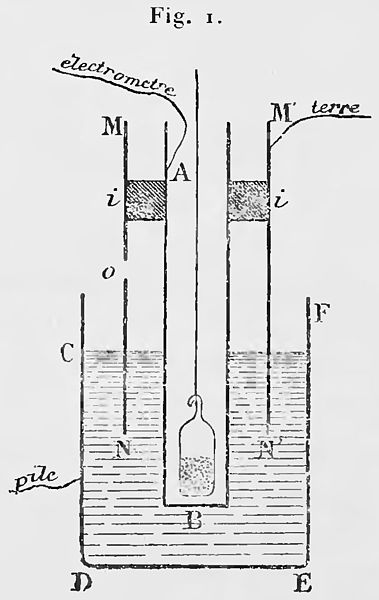
Marie Curie worked with an instrument called the "electrometer" invented by Pierre Curie, this apparatus consists of detecting very weak electrical currents, and with this apparatus Marie was able to discover something extremely surprising, and that is that by exposing the uranium salts to the air and with the help of this apparatus she was able to detect that "the radiation emitted by the uranium ionized the air, that is to say the air became a conductor of electrical current", for that time it was a surprising discovery. To confirm this, he removed the uranium salt exposed to the air and was able to determine that the air returned to its natural non-ionized state. Qualitative observation was of utmost importance in this experiment, but he managed to widen the gap by measuring the intensity of the radiation emitted in a quantitative way, all this by substituting the salt with another type of material, and thus being able to verify by means of the electrometer the ionization circulating in the air in order to quantify the activity of the material. In this way, Marie was able to understand that what was important in Becquerel's work was uranium, since it was the cause of this ionization.
Uranium was the source of active radiation that can ionize the air. Physicists at that time could not believe that within all this a chemical phenomenon caused by uranium atoms was occurring, no matter what other substance or material was present, uranium spontaneously emitted this radiation. The change was negligible and could not be seen with the naked eye in the uranium atoms which caused the uranium to be an active source of radiation.
After this, different experiments were carried out with various substances to prove that it was not only uranium that was radioactive, but after so many experiments they did not get others with the properties of uranium. Only one substance has similar but not as powerful properties as uranium, this substance was discovered by Gerhard Schmidt which was "thorium". Then Marie was able to experiment with this substance and discovered something very important by conducting experiments with a mineral composed of uranium oxide "pitchblende" and the surprise was that this mineral was more active than the same fascinating uranium!.
But why was this ore more active than uranium?
Just at that moment Marie thought that something must be in this rock to offer these fascinating properties. It was here that her husband decided to abandon everything he was doing in order to join with his beloved Marie and work together on this discovery. They dedicated all their time and effort in their small laboratory in order to decipher what was so small and powerful that contained this mineral that made it more radioactive than uranium.

Pierre and Marie Curie in the lab. CC BY-SA 3.0
So they both decided to completely remove the super radioactive substance from the pitchblende. The work was not easy because the concentration of this rock was so small that it made very large amounts of pitchblende. In order to detect this agent that caused the radiation, it required a subtle hand in the chemical processes and to be able to detect this phenomenon. But it was so complicated to find this radioactive substance that during the many attempts in their experiments they managed to find another one totally different from the one they expected during the chemical processes to isolate it, so it was not one single substance but more than one although with different properties. Then in 1898 they both discovered a new element by isolating another one and called it "Polonium". At the end of that same year, after multiple attempts during the chemical processes, they managed to isolate the small, most radioactive substance and named it "Radium".
In order to achieve the discovery of this element, approximately 1000kg of pitch blende was needed, since the concentration of radium was negligible, but its instability was very great, perhaps 2 million times more than uranium, which makes it difficult to find.
Working with this type of element was extremely dangerous, taking into account that at that time the safety measures were practically non-existent, which meant that both Marie and Pierre were totally exposed to radiation during their experiments which in the end after a few years could take its toll. This was the cause of Marie Curie's death because she was exposed to radiation for so long.
However, the discovery of all these elements does not answer the questions left unanswered by Becquerel's work. We were still in doubt as to why these substances or elements emit radiation? what was the cause? and the other, what exactly was this radiation?.
It took decades to be able to answer the first question and it would be quite extensive if I explain this answer step by step, for which I will dedicate another publication. But the second question is a little easier to answer.
The physicists were able to realize something very interesting and that is that the elements did not emit the same type of radiation that Becquerel had discovered. How so? After several experiments they realized that they emitted three different types of radiation.
you know where I want to go, right? :)
To the X-rays discovered by Röntgen, we had to add another 3, therefore the Becquerel rays would no longer be called as Marie Curie had called them at the beginning of her work, but as it was still not very well known about the properties that these 3 types of rays had, this type of radiation was assigned arbitrary names called alpha(α), beta(β) and gamma(γ).
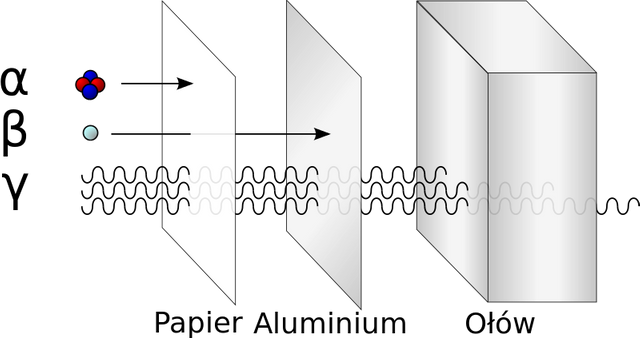
The rays α were electrically charged particles that were produced when they passed through a fairly powerful magnetic field so that they could be visualized (not with the naked eye) simply by detecting them and noticing the deviation. This radiation was absorbed by substances very quickly so they had a very small power of penetration as for example a piece of paper.
The rays β also present the same particularity as the previous ones since when crossing a magnetic field they can deviate, unlike these made it much easier and with less charge and mass in its particles and of course the most important charges are negative as opposed to the alpha ones that have positive. When experimenting with this type of radiation it was possible to observe that in order to detect them a not so thick metallic plate is needed and when carrying out the measurements of the charge-mass ratio of these particles it was possible to conclude that they were very similar to the cathode rays produced in the tubes that Röntgen used.
And the third type of radiation whose name is gamma γ was the opposite of the previous two radiations because it did not deviate when passing through a magnetic field. The behaviour of its particles was found to be similar to the X-rays originally discovered by Röntgen.
In fact, it was found that according to the substance with which the person was observing, it produced a different type of radiation, that is, some of these 3 types of rays mentioned above. For example, radium emitted all 3 types of radiation, uranium emitted beta and gamma, and alpha and gamma polonium. Why did this happen?.
The answer was simple at the time: they didn't have the slightest idea hahaha.
Pierre Curie mentioned that the amount of heat that was led by the radius is enormous can be compared to any chemical reaction with a proportion equivalent to matter. Impressive. But such heat only appeared when energy was involved in transforming a small proportion of the radius that could not be seen.
What this meant: "that the transformation could go much further than they imagined at that time, that is, much further than common and ordinary chemical transformations, could be in a possible presence of elemental transformation that could be because of the amount of heat involved in the elements themselves which in turn needed transformation at the atomic level. For this reason they continued the experiments with these elements and observed the appearance of new elements that previously were not in the chemical processes, this was a very important step a clear example of this is that when experimenting with radium another element could be produced like helium", as well as from nothing.
In order to translate all this, the basic principles of chemistry were being violated, for the simple fact that when you experiment with one element it disappears and another new element is born. But for some unknown reason, the element was being transformed at the atomic level into others and releasing various radiations of tremendous energy. This energy could turn these substances into some dangerous agent due to their instability.
In those times the power of a radioactive element was not known, that's why the scientists carried out their experiments without taking preventive measures, they didn't have knowledge of the damage to the health that could cause an element like the radium or uranium, It is like for example that Pierre Curie took a piece of uranium and put it in his pocket as if it was a personal belongings or some kind of ornament, as days went by he could realize that his skin presented a red color and felt burning and his skin later burned, this is due to the little knowledge they had at that time for being something totally innovative for that time. That is why nowadays this type of radioactive elements are kept in a kind of lead shield so that the radiation cannot spread and thus avoid side effects on people's health.
In my next installment I will talk more about these X-rays and thus be able to clarify all the questions that these scientists of the time raised. I hope you enjoyed my content.
Don't miss my next installment. See you later.
Content support
Publish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg

This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Thank you ;)
Congratulations @carloserp-2000! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard: