Laws of chemical combination #2 : Law of definite proportion
Hello steemians, this is the second post of series of posts on "laws of chemical combination". I have already posted about law of conservation of mass. In this article, I am going to write about law of definite proportion.

(www.shutterstock.com)
Law of definite proportion
This law was first put forward by French chemist Louis Proust in 1799 AD. It states that, “In same chemical compound, same elements are present which are combined in fixed weight composition irrespective of source and mode of formation.”
This law can be explained by following illustrations.
Illustration 1
Carbon dioxide is found in all countries. If the pure sample of carbon dioxide is taken from different countries, its chemical formula is same CO2. The carbon and oxygen combine in the fixed ratio 12:32 or 3:8 irrespective of country.
This verifies law of definite proportion.
Illustration 2
Carbon dioxide can be prepared by various methods as shown:
a. Calcium Carbonate  Calcium Oxide + Carbon dioxide
Calcium Oxide + Carbon dioxide
b. Calcium carbonate + Hydrochloric acid  Calcium chloride + Carbon dioxide + water
Calcium chloride + Carbon dioxide + water
c. Carbon + Oxygen  Carbon Dioxide
Carbon Dioxide
If the samples of carbon dioxide formed by different preparation methods are checked, there would be definite composition of carbon and oxygen to form carbon dioxide. i.e in the ration 3:8 by weight.
There are the conditions where law of definite proportion doesn’t hold good. They are as follows
a. If different isotope of element is used.
If 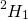 is used, H2O has hydrogen to oxygen ratio 1:8 by weight
is used, H2O has hydrogen to oxygen ratio 1:8 by weight
If 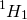 is used, H2O has hydrogen to oxygen ratio 1:16 by weight
is used, H2O has hydrogen to oxygen ratio 1:16 by weight
So, different isotopes of same element give different proportion in chemical combination
b. There are certain compounds called non stoichiometric compounds or Berthollite compounds, which don’t follow law of constant proportions. Eg. ZnO, CuS, FeS etc.
Example:
- Quicklime contains 71.47% of calcium. How much calcium is present in a sample of quicklime which contains 16 g of oxygen?
Solution:
Quicklime contains 71.47% calcium
So, percentage of Oxygen = (100-71.47) % = 28.53 %
Let total weight be x
Then 28.53% of x = 16 g
Or, x= 56.08 g
The weight of calcium = 56.08 – 16 = 40.08 g
References
Steemstem:
SteemSTEM is a community driven project which seeks to promote well-written and informative Science, Technology, Engineering and Mathematics posts on Steemit. The project involves curating STEM-related posts through upvoting, resteeming, offering constructive feedback, supporting scientific contests, and other related activities.
DISCORD: https://discord.gg/j29kgjS
Part 1 of this article.
Laws of chemical combination #1: law of conservation of mass

Congratulations @bikkichhantyal! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThis post has received a 4.93% UpGoat from @shares. Send at least 0.1 SBD to @shares with a post link in the memo field.
Invest your Steem Power and help minnow at the same time to support our daily curation initiative. Delegate Steem Power (SP) to @shares by clicking one of the following links: 1000 SP, 5000 SP or more. Join us at https://steemchat.com/ discord chat.
Support my owner. Please vote @Yehey as Witness - simply click and vote.
Great post! You just got a 9.06% upvote from @edensgarden!
Thanks for tasting the eden!
You got a 4.83% upvote from @mitsuko courtesy of @bikkichhantyal!