Two-dimensional materials: Graphene
In 2010 Geim and Novoselov received the Nobel Price "for groundbreaking experiments regarding the two-dimensional material graphene." After this event, a large amount of research was done to isolate other two-dimensional materials and find out about the characteristics of these thin layers of atoms. Having these unique properties, 2-D materials can be used in many different forms of application such as semiconductors, optoelectronics and batteries.
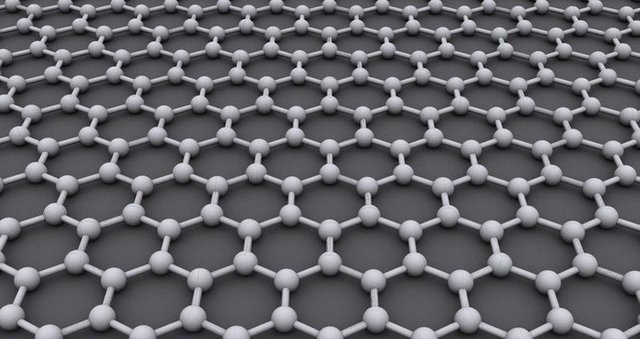
Carbon
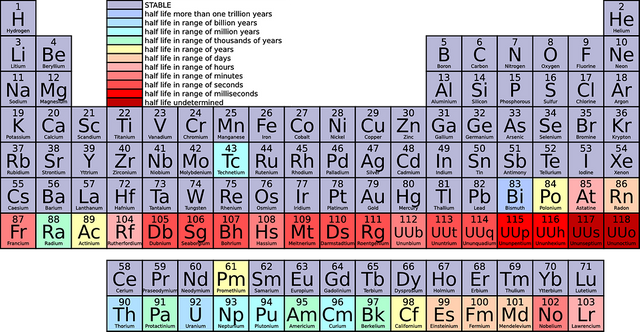
As graphene consits out of carbon, I want to shortly summarise some facts about it. Carbon is an essential element of our biosphere. After oxygen it is the (measured by weight) often existing in creatures. It's chemical sign is "C" an is labeled with the atomic number 6, meaning 6 protons are found inside the nucleus of the atom. You can find it in the periodic table, where it is localised in the 4th main group (carbon group) and in the 2nd period:
Carbon has the ability to occur in different modifications, meaning the different forms are only built out of carbon and no extra atoms e.g. hydrogen. A diamond would be one of these allotropes. Looking at how the atoms are arranged, we can see that they are arranged tetrahedrally with one carbon atom binding to four other carbon atoms. The second modification would be Graphite, being a crystalline allotrope with a layered, planar structure of which one layer is what we call graphene. The last two allotropes are fullerenes and nanotubes. To summarise it, fullerenes are build out of 5 and 6-rings, forming a soccerball- like structure. You can imagine nanotubes like a tube out of carbon, kind of like rolling a graphene sheet. Because this all sound kind of weird, let's take a look at a picture:
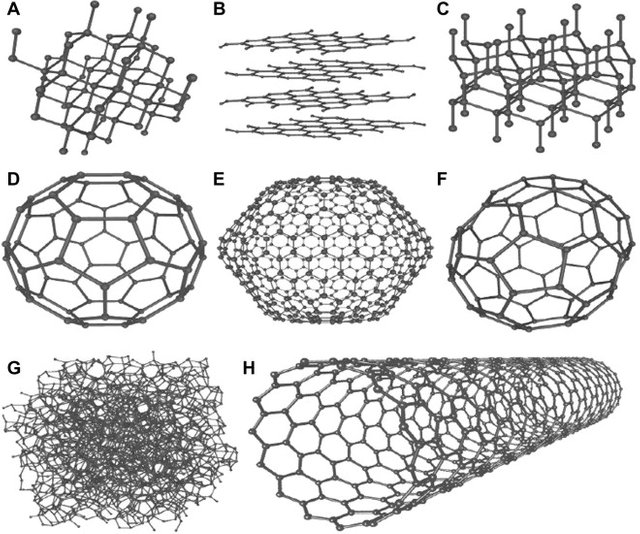
The picture is showing 8 allotropes, but the one's mentioned above are: A (Diamond), B (more than one layer= Graphite, one layer= graphene), C (Fullerene) and D (nanotube)
Graphene
The allotrope we want to look at now is garphene. Graphene is a modification of carbon consisting out of a two-dimensional, 0.345Nm thick and sp2-hybridised (s-orbital connects with two p-orbitals) hexagonal structure where each atom is covalently bond to three other atoms, each with the distance of 142 picometer (pm):
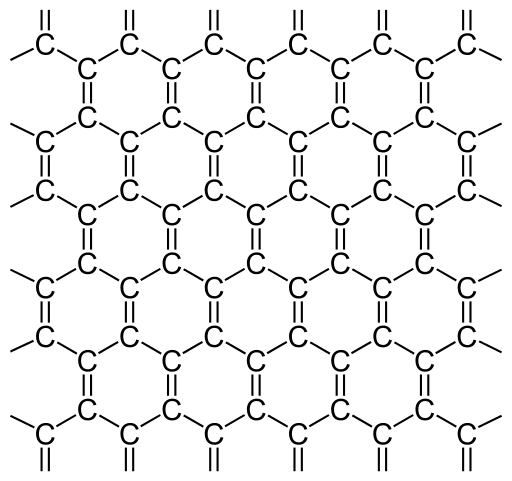
Structure of graphene. One C-atom is bound to three other C-atoms
If we stack these graphene layers on each other we get a material of our daily usage: Graphit. The crystalline allotrope of carbon we use for our pencils is just consisting of these 2d structures put on top of each other and hold together by Van-der-Waals-forces.
Are you sometimes bored during school/work and have a pencil near you? Then you just need some duct tape and you can get your own graphene. As simple as it sounds, this was exactly the method the scientists used (maybe a little bit more precise ;) ). The process is called exofoliation and can be done with e.g. plastic tape or a chemical reaction.
If this doesn't work out for you, just put it into your mixer and put a solvent or dish soap into it and voilà: graphene. Although this may sound like we could already produce tons of graphene, these methods only create small flakes which maybe are suitable for transistors, but for bigger products other methods are necessary. Mass production methods are currently under worldwide research. Scientists from the Kansas State University (KSU) developed a process, using a chamber filled with acethylene/ethylene gas & oxygen and then creating a contained denotation through a spark plug. Graphene can in general either be created top-down (exofoliation...) or bottom-up (for example growing it on polycrystalline copper foil).
Special porperties
Graphene's electrical conductivity is reported above 15000 cm2⋅V−1⋅s−1 with limits up to 200000. Comparing it to copper, it is way more conductive and has way better mobility for the electrons inside. The reason for the conductivity is a missing band gap:
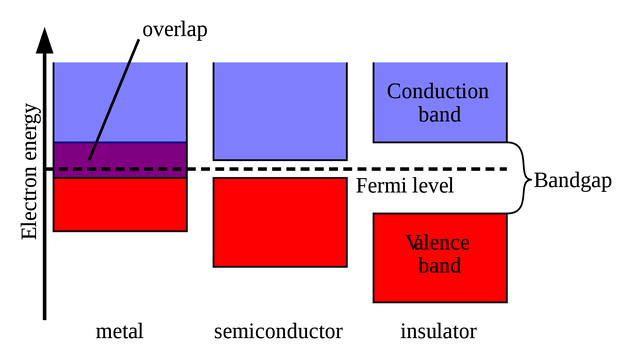
Band gaps determine the electrical conductivity
The lower graphic illustrates where the valence and the conduction bands of graphene connect at the six dirac points, forming a zero-gap semiconductor which is the reason for it's property of being conductive:
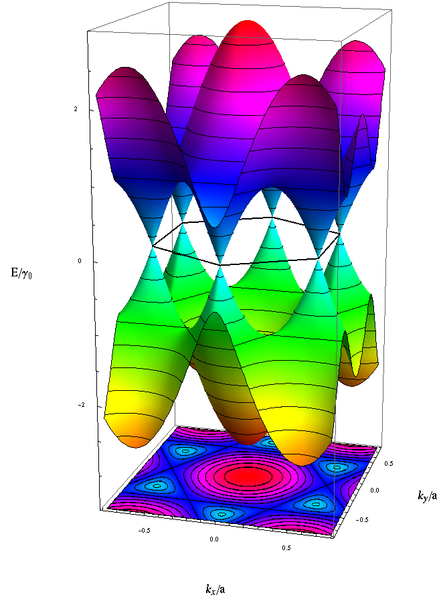
The tight band model
Beyond the electrical conductivity its high thermal conductivity and high intrinsic strength from about 130 gigapascals as a result of the covalent bonds, it opens the doors for many applications. For freely suspended samples, the in-plane thermal conductivity of graphene at room temperature is among the highest of any known material, about 2000–4000 W m –1 K –1. In the 2-dimensional room, the phonons in the graphene nearly lose no energy transferring the heat. These properties are contradicting Fourier's law because the larger the segment of graphene, the more heat it could transfer. This proves, the thermal conductivity isn't an intrinsic material constant value.
How can graphene help the industry & consumers?
You can already guess of wide the range of possible uses for such an unique material are. Graphene sensor can be used in biological engineering to build sensors, monitoring blood sugar levels or diseases. The materials sensitivity is so high, it can already detect single molecules of gas, making it suitable to detect toxic gases or chemicals. Bridges, aircrafts and other constructions can use these sensors to collect data about the stability and impacts on different areas, improving overall security.

Besides sensors, energy storage is a field graphene can help to create better products. Mini-supercondensators for smartphones and other little devices aswell as lithium-ion batteries with an graphene anode are an example of incorporating graphene to increase energy storage. Solar panels can also profit from the conductivity and high transparency as they need materials with these attributes to work well. Doping materials with small amounts of graphene can already enhance their conductivity and strength. Many composite materials can be corporated into already existing systems. Aircrafts for example can reduce fuel consumption by replacing steel components with the strong and light graphene to reduce fuel consumption.

As we are already talking about reducing emissions and costs, improving the costs of LED displays by replacing indium-based electrodes in OLEDs with graphene also helps to make it easier to recycle as no metals have to be used. Filtration is another field of application. Membranes of garphene oxide are impermeable for all gases and vapors besides water, making it a very useful for filter systems. Even helium, the hardest gas to block can be stopped through the 2d material, implying it's potential. Heat could be transported effectively using it's thermal conductivity abilities, eliminating the limiting factor for smaller and more efficient components. Drug carrying medical systems could be developed... and the list goes on.
Why we aren't already having these cool products ( + conclusion) ?
Although products like tennis rackets are already under the use of champions, using ultra fast batteries and processors including graphene in a smartphone while walking towards your perfectly working electric car due to the graphene membranes in it isn't likely to happen in a few weeks.

Mass production methods are currently under development and have to be approved to work correctly and produce high-quality graphene without cracks. Comparing it to steel, garphene is also very complex to fabricate into many shapes. You can add many more example here from computer chips to the transfer it perfectly without any defects. The problem with such materials is expecting miracles. Just like every new material, graphene gives companies many possible uses for it. Finding solutions to these problems, targeting the right field of application and continously developing new methods of producing it are necessary. Day by day more research is done and more combinations of graphene with other materials promise upgraded products. Safety of the material for the human organism on nanoscales a growing industry will be the basis for upcoming technologies that hopefully improve our products and sustainability and shrink the costs of it.
Thanks for reading and have a nice day :)
Source
Texthttps://en.wikipedia.org/wiki/Graphenehttps://www.fkf.mpg.de/54379/kk634.pdf (translated)http://www.dummies.com/education/science/nanotechnology/graphene-sheets-of-carbon-based-nanoparticles/http://poplab.stanford.edu/pdfs/PopVarshneyRoy-GrapheneThermal-MRSbull12.pdfhttps://www.graphenea.com/pages/graphene-properties#.Wm3Nya7iYuVhttps://academic.oup.com/nsr/advance-article/doi/10.1093/nsr/nwx055/3861355https://www.k-state.edu/media/newsreleases/2017-01/graphenepatent12517.htmlhttps://www.engadget.com/2017/01/30/explosions-may-be-the-answer-to-mass-producing-graphene/https://www.nobelprize.org/nobel_prizes/physics/laureates/2010/https://phys.org/news/2014-05-physicists-unlimited-graphene.htmlhttp://www.graphene-enabled.com/applications/http://www.escapistmagazine.com/articles/view/scienceandtech/15469-5-Applications-and-Uses-for-2D-Materials.4https://www.wired.de/collection/science/warum-setzt-sich-das-wundermaterial-graphen-eigentlich-nicht-durch (translated)https://de.wikipedia.org/wiki/Graphen (translated)https://de.wikipedia.org/wiki/Kohlenstoff#Graphit-Struktur_(sp2) (translated)http://www.spektrum.de/lexikon/physik/fullerene/5389 (translated)https://www.nanoscience.com/applications/education/overview/cnt-technology-overview/http://www.chemie.de/lexikon/Kohlenstoff.html (translated)https://www.welt.de/wissenschaft/article116596053/Warum-der-Superakku-doch-ziemlich-unsuper-ist.html (translated)https://en.wikipedia.org/wiki/Potential_applications_of_graphene#Sensorshttps://www.graphene-info.com/graphene-solar-panelshttp://www.understandingnano.com/graphene-applications.htmlhttps://www.uk-cpi.com/blog/13-graphene-applications-that-will-transform-your-futurehttps://www.azonano.com/article.aspx?ArticleID=3492https://www.graphene-info.com/graphene-applicationshttp://www.graphene.manchester.ac.ukhttps://www.azom.com/article.aspx?ArticleID=14154https://arxiv.org/ftp/arxiv/papers/1301/1301.6181.pdfPictureshttp://www.dynatec.es/blog/wp-content/uploads/2017/01/grafeno-660x350.jpghttps://cdn.pixabay.com/photo/2012/04/26/11/17/periodic-table-42115_960_720.pnghttps://www.researchgate.net/profile/Ahmad_Aqel/publication/230777261/figure/fig18/AS:268043242700834@1440917897559/The-structures-of-eight-allotropes-of-carbon-A-Diamond-3D-network-covalent.pnghttps://upload.wikimedia.org/wikipedia/commons/thumb/7/71/Graphene_structure.svg/512px-Graphene_structure.svg.pnghttps://upload.wikimedia.org/wikipedia/commons/thumb/c/c7/Isolator-metal.svg/2000px-Isolator-metal.svg.png/https://upload.wikimedia.org/wikipedia/commons/thumb/0/0b/GrapheneE2.png/439px-GrapheneE2.pnghttps://www.roadtraffic-technology.com/wp-content/uploads/sites/17/2017/10/1-image-23.jpg://www.pavablog.com/wp-content/uploads/2017/04/AVIAO-1000x600.jpghttps://c1.staticflickr.com/1/602/21296670609_87c49fb6b7_b.jpg