Experiment shows a reversed entropy effect
Hi everyone :)
If we think about the second thermodynamics law, we know a cup of hot tea will heat up the air around, while it's own temperature decreases. The law states, heat spontaneously moves from hot to cold bodies. An experiment proved what scientists already expected. They changed the temperature of two nuclei in two of the atoms that exist in a molecule of trichloromethane—hydrogen and carbon, so that the temperature of the hydrogen nucleus was higher than the carbon nucleus. A strong magnetic field aligned the nuclei and radio pulses then flipped one or both spins, causing them to become correlated.
Results of the experiment
When the energy states were uncorrelated, an heat flow like we would expect occurred: from the hydrogen to the carbon. Now, as they were correlated (meaning they are in a state where they share information which isn't possible for lager objects, but less strong than entanglement), the opposite happened: The hot nucleus grew hotter while the cold grew colder, showing the opposite of what observing a classical system would point out.
If you want an exact overview about the experiment, follow this link: https://arxiv.org/pdf/1711.03323v1.pdf
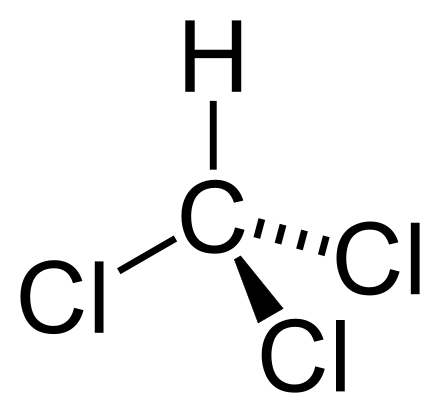
trichloromethane
The second law of thermodynamics...
is referring to the fact, the total entropy of a system can never decrease over time, but two systems can be in equilibrium, meaning ∆ system1 + ∆ sytem2 = 0 . It includes the fact that heat can't be 100% transferred into mechanical work (e.g. engine of a vehicle), so the more we transfer it, the more energy is getting wasted. Nature will also strive into a state of higher dissorder and there is no perpetual motion machine of the second kind. From the law we can also figure out, heat can only flow from a hot to a cold body and not the other way around.

an example of thermodynamics in our daily life: the engine heats up if we use it, having a specific energy conversion efficiency
Does this experiment violate thermodynamic's second law then?
No, it doesn't. "The standard second law in its local form apparently fails to apply to this situation with initial quantum correlations." Short story, the second law applies only on average to large objects and doesn't consider quantum correlation. Still, the second law is verified for the isolated two-spin system. It doesn't consider initial correlations between interacting systems and this case was already predicted by Boltzmann. What the team did, was to build up a system with initial conditions which force the mirroring of the heat flow. In our universe, the initial conditions force entropy to increase, so we will never see a broken glass reassembling itself from alone.
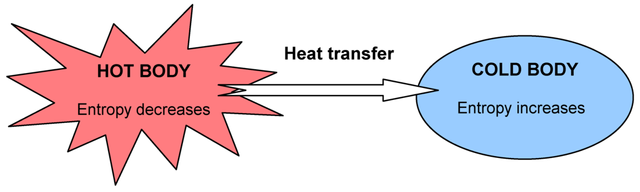
Conclusion
The experiment isn't the start of a revolutionary method to travel back in time, but it is proving already existent assumptions and gives us a better understanding of how thermodynamics work in a prepared system. Such research could open the way to control the heatflow on microscale, enabling to find out if it's possible to use it for applications like electrical devices. The team also states, their experiment could consequences on the cosmological arrow of time.
Thanks for reading and feel free to share your opinion about the experiment :)
Have a nice day :)
SourceTexthttps://arxiv.org/pdf/1711.03323v1.pdfhttps://phys.org/news/2017-12-arrow-relative-concept-absolute.html https://www.technologyreview.com/s/609788/physicists-demonstrate-how-to-reverse-of-the-arrow-of-time/ http://www.pbs.org/wgbh/nova/next/physics/scientists-reverse-arrow-of-time-in-quantum-experiment/ https://www.sciencenews.org/article/arrow-of-time-reversed-quantum-experiment https://www.livescience.com/50941-second-law-thermodynamics.html https://www.lernhelfer.de/schuelerlexikon/physik-abitur/artikel/zweiter-hauptsatz-der-thermodynamik (translated) https://en.wikipedia.org/wiki/Second_law_of_thermodynamicsPictureshttps://upload.wikimedia.org/wikipedia/commons/6/67/Varsha_ys.jpghttp://upload.wikimedia.org/wikipedia/commons/thumb/9/9f/Chloroform_displayed.svg/440px-Chloroform_displayed.svg.png https://cdn.pixabay.com/photo/2012/02/29/12/05/car-18847_640.jpghttps://upload.wikimedia.org/wikipedia/commons/c/c3/Entropy_Hot_to_Cold.png
Congratulations @aximot! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP