The chemistry behind burnt food
In order to avoid burn crusts or damage the flavor of your dish, you must handle tempereature and cooking time carefuly. And you probably realized that sweet products and mixes are proner to burn faster than unsweet ones. Why does this happen? Because reducing sugars (and amino acids) are raw material to the happening of Maillard Reaction, and if there are more reactives, there will be more products.

Overcooked Pumpkin Pie. Source
What is "Maillard Reaction" about?
Maillard Reaction is a process that occurs at high temperatures and low water activity. It happens when a reducing sugar like glucose, galactose or maltose reacts with an amino acid joining its anomeric carbon (the one with he carbonyl group) to the amino group and result in the formation of a molecule of water and a N-substituted glycosylamine.
The glycosylamine is unstable so it will experience a rearrangmet to form a ketosamine that can react further and end as a reductone or a melanoidin. This last kind of chemicals is responsible of giving the toasted food its characteristic texture, color and flavor.
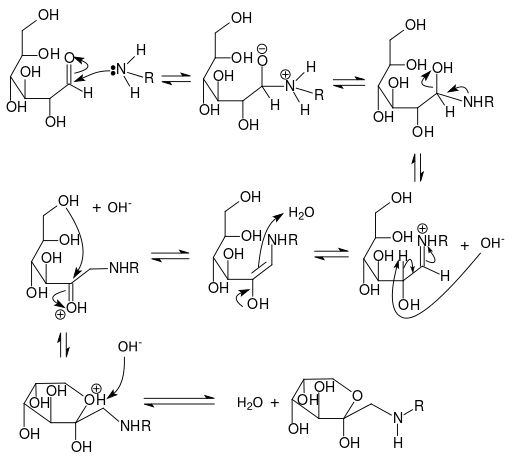
Mechanism of the Maillard Reaction. Source
Why do we have to keep an eye on Maillard Reaction?
Even when the toast flavor is tasty and delightful, Maillard Reaction needs to be controlled. This reaction is irreversible and makes the amino acids biollogically unavailable, hence, it reduces the nutritional value of the food. Also, there is an incredible amount of amino acids in food such as asparagine which Maillard product is acrylamide, a hazardous chemical compound known for its carcinogen properties.
Sources
- Chichester, C. O., ed. (1986). Advances in Food Research. Advances in Food and Nutrition Research. 30. Boston: Academic Press. p. 79. ISBN 0-12-016430-2.
- Melanoidines
- Acrylamide
Other information was taught to me by Félix Millán, teacher of "An Introduction to Food Industry Technology" course at Universidad Simón Bolívar which I attend to.
Disclaimer
This is my first contribution to the #steemstem community. Sadly, I'm not able to use the the steemit chat due to some sign up problems. But if there's another way to be in contact with you, please let me know in order to keep the divulgation of interesting scientific topics.
Hope you enjoyed the read.
Interesting!
Hola @aristidesp, upv0t3
Este es un servicio gratuito para nuevos usuarios de steemit, para apoyarlos y motivarlos a seguir generando contenido de valor para la comunidad.
<3 Este es un corazón, o un helado, tu eliges .
: )
N0. R4ND0M:
9546 6224 5232 6533
1328 9121 1890 9755
9665 1586 6311 9770
8260 1010 5310 6752
Congratulations @aristidesp! You received a personal award!
Click here to view your Board
Do not miss the last post from @steemitboard:
Congratulations @aristidesp! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!