Polycyclic Aromatic Hydrocarbons in Foods
Greetings, dear readers.
In this post I share some considerations about a type of contamination of fossil sources that is present in our homes by the preparation of food.
Introduction
When my professor of environment, development and health in one of her classes began to introduce the subject of Polycyclic Aromatic Hydrocarbons, my attention was caught by the barbecue when she pointed it out among the pyrogenic sources that generate these carcinogenic substances.
In a family sharing where meat, chicken and their rich derivatives are being roasted, millions of biomolecules composed of carbon, hydrogen and oxygen can be produced (to a lesser extent), without downplaying the importance of soot and tar by the combustion of dry wood and coal. My preference for cooking charcoal-grilled meat led me to be interested in knowing this information and sharing it.

When cooking meat at high temperatures or direct fire potential carcinogenic substances are generated, among them Polycyclic Aromatic Hydrocarbons.
Meanwhile, I turned my attention to the studies of Bloch and Dreifuss, who in Zurich (1921) discovered substances with traces of tar responsible for generating malignant tumours in people who had been exposed for considerable periods of time to these compounds, characteristic of the group of polycyclic hydrocarbons.
Years later, the British pathologist Sir Ernest Laurence Kennaway validated his theory of obtaining carcinogenic compounds from the exposure of petroleum, isoprene, acetylene, yeast, cholesterol and even animal tissue and skin to thermal decomposition processes (pyrolysis) and high temperatures.
The pyrolysis of organic matter is a very extended thermochemical process, it is given by lack of oxygen, which implies an incomplete combustion. This anomaly causes the formation of harmful by-products such as carbon monoxide (CO) and the so-called PAH; once the pyrolysis process has been completed, a wide distribution of PAH comes into play in all the components and environmental elements: air, soil, water, sediments, biological tissues, including some foods. At this point, it is of concern for biological diversity and human health.
Subsequent studies will conclude and would serve as a compass to hold benzo[a]pyrene (3, 4-benzopyrene in the old nomenclature) responsible as a potent tumourigenic agent of tar and other fluorescence characteristics that were being investigated in certain fractions.

Polycyclic Aromatic Hydrocarbons
It turns out that this family of compounds spreads daily throughout the environment; they are characterized by being polynuclear structures that manifest photochemical destabilization, or rather, they are susceptible to degradation with light (photooxidation). They originate from the bad combustion of any organic matter whether by natural process, industrial, domestic, transport, tobacco, smoked meat and fish, among others, as stated by the Agency for Toxic Substances and the Registry of Diseases:
Polycyclic aromatic hydrocarbons (PAHs) are a group of over 100 different chemicals that are formed during the incomplete burning of coal, oil and gas, garbage, or other organic substances like tobacco or charbroiled meat. PAHs are usually found as a mixture containing two or more of these compounds, such as soot. Source
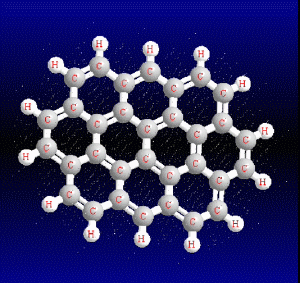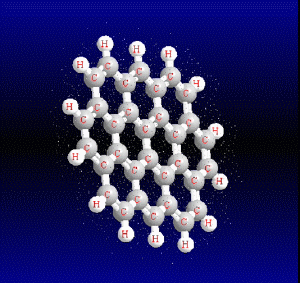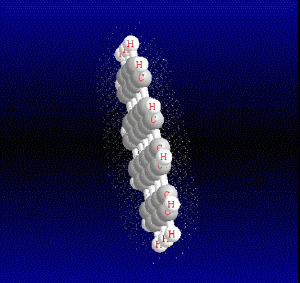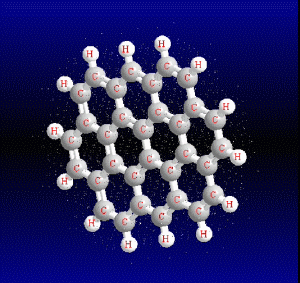
Chemical structure of the main PAHs
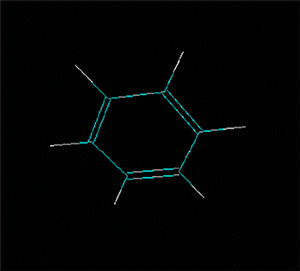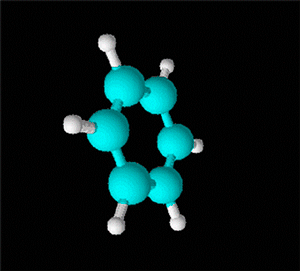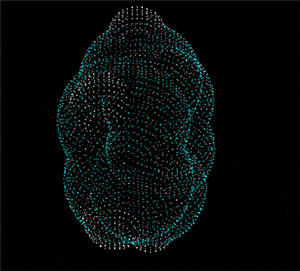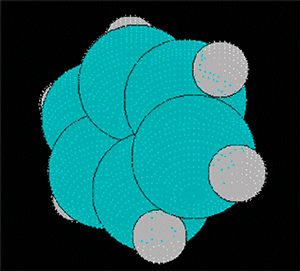
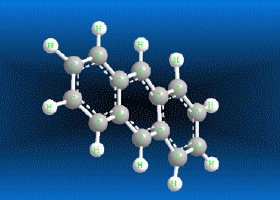
Polycyclic aromatic hydrocarbons are compounds with a structure of several benzene rings that have been unified by a complex biochemical process. Each vertex of the molecular structure corresponds to a carbon atom, this has 4 links represented by the segments that are interconnected to each vertex; in most of them 3 links are visualized, which means that the remaining link is occupied by a hydrogen atom. As you will see in the animated illustration, the chaining of benzene rings underpins its polycyclic condition.
Data retrieved from here
Benzene is a hydrocarbon whose molecular formula is C6 H6 with a characteristic ring appearance, complements its name of benzene or aromatic ring, its smell of benzene. Another interesting aspect is that in this molecular compound each carbon atom occupies the vertex of a regular hexagon, as seen in the three-dimensional image, the cyan color represents carbon, while the white color identifies hydrogen. The main polycyclic aromatic hydrocarbons with a description of their chemical properties are shown below.

Molecular formula: C14 H10 Density: 1280 kg/m3; 1,28 g/cm3 Melting point: 488,8 K (216 °C) Boiling point: 613 K (340 °C)

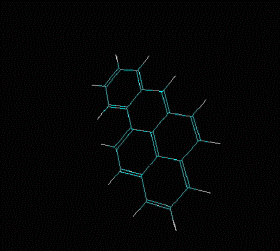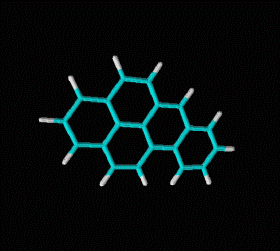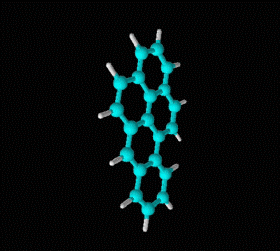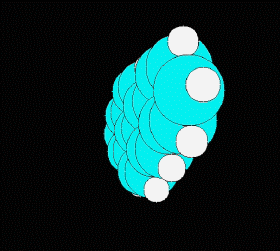
Fórmula molecular: C20 H12 Density:1240 kg/m3; 1,24 g/cm3 Melting point: 179 K (452 °C) Boiling point: 495 K (768 °C)

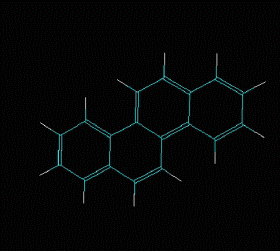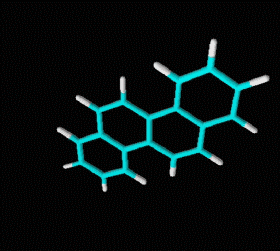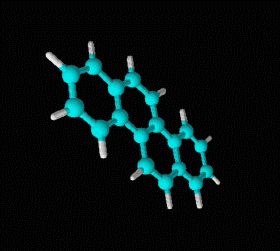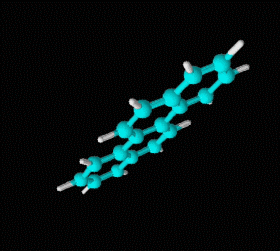
Fórmula molecular: C18 H12 Density: 1274 kg/m3; 1,274 g/cm3 Melting point: 527 K (254 °C) Boiling point: 721K (448 °C)

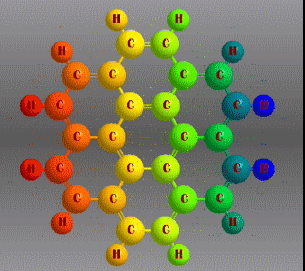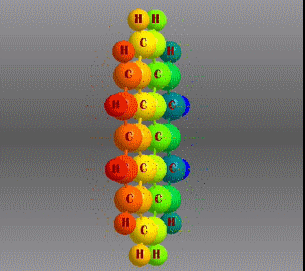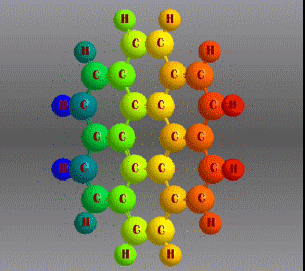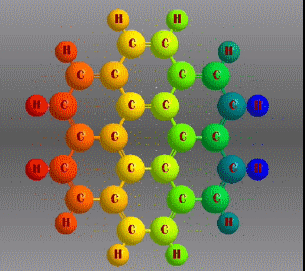
Fórmula molecular: C24 H12 Density: 1.371 g/cm3 Melting point: 437.3 °C (819.1 °F; 710.5 K) Boiling point: 525 ° C (977 ° F; 798 K)
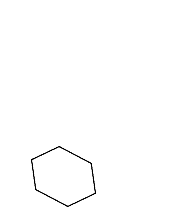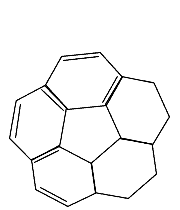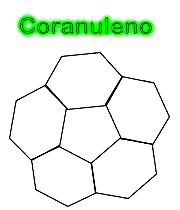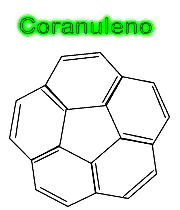
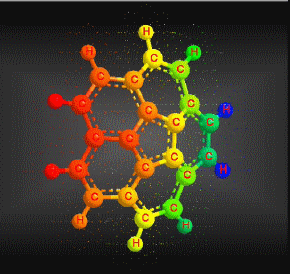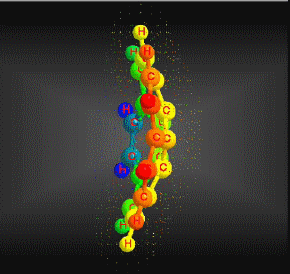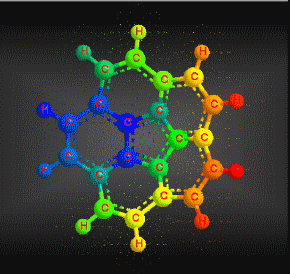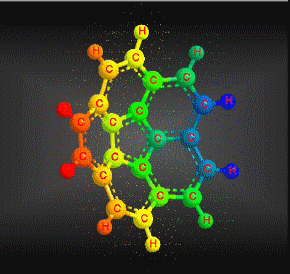
Molecular formula: C20 H10 Molar mass: 250.29 g/mol

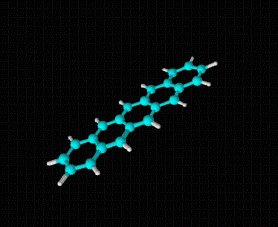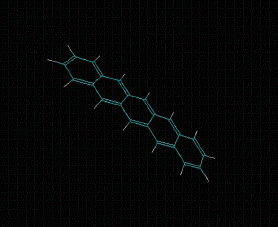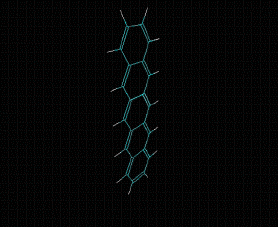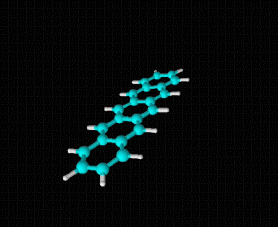
Molecular formula: C24 H12 Density: 1.3 g/cm-3 Melting point: >300 °C/sublimates 372 ºC Boiling point: 40 - 43 ° C

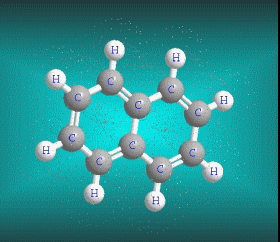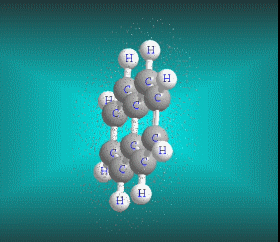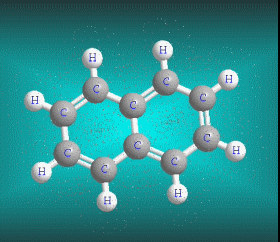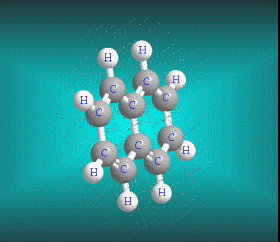
Molecular formula: C10 H8 Density: 1.45 g/cm-3 (15.5 ºC) Melting point: 78.2 ° C (172.8 °F; 351.3 K) Boiling point: 217.97 ° C (424.35 °F; 491.12 K)

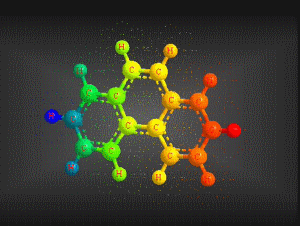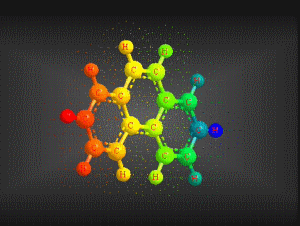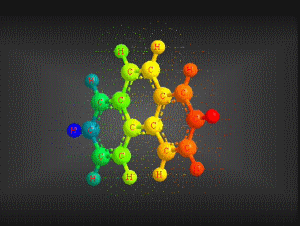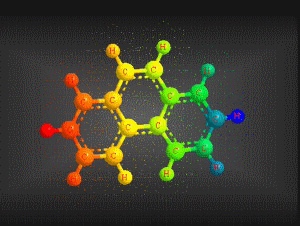
Molecular formula: C14 H10 Density: 1180 kg/m3; 1,80 g/cm3 Melting point: 374,15 K (101 °C) Boiling point: 613,15 K (340 °C)

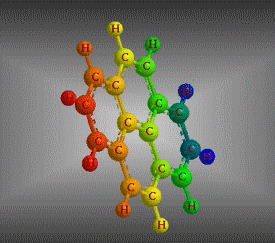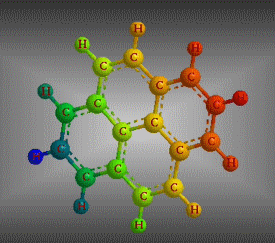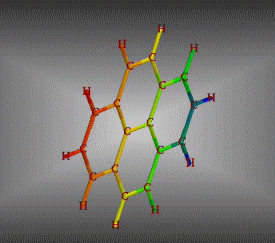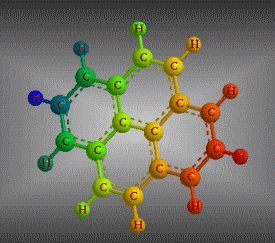
Molecular formula: C16 H10 Density: 1271g/cm3 Melting point: 0.135 (a 25 ° C) Boiling point: 393.5 ° C (666.65 K)

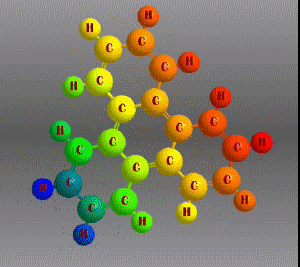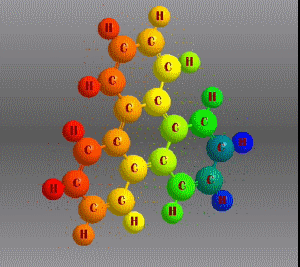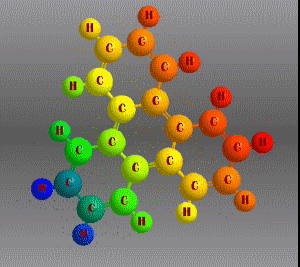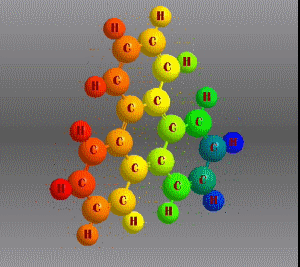
Molecular formula: C18 H12 Density: 1,302 g/cm3 Melting point: 199 °C Boiling point: 438 °C (1013 hPa)

Molecular formula: C32 H14 Density:1,302 g/cm3 Masa molar: 398.45 g/mol Scarcely soluble in solvents such as benzene, toluene and dichloromethane.

PAHs as Environmental Pollutants
There are diverse sources of PAH emissions on a global scale, although a significant part comes from natural processes, the main sources are the industrialized centers and urban dynamics; in the latter are those related to transport services, heating, burning of garbage and incineration of synthetic waste.
It was similar in the 1990s in Germany, where low temperatures were accompanied by significant amounts of benzo[a]pyrene from the burning of coal as a heating mechanism, while another considerable volume originates from industrial activity and automobile fleets.

PAH in food
Among the main exogenous sources of genotoxic compounds are food and tobacco. In the case of benzo[a]pyrene, its mutagenic properties and its direct impact on health through the generation and reproduction of carcinogenic cells are striking.

Process of accumulation and release of PAH by segregation and burning of fat in meat.
There are many ways in which PAH can be produced, taking up the anecdotal development of these chemicals by grilling, it happens that subjecting meat and its derivatives to cooking with firewood, charcoal and even frying in frying pan at high temperatures, makes foods produce large amounts of fat and fat-soluble juices that, When in contact with fire, vapors and smoke charged with PAH emanate and partially adhere and accumulate in food, which are generally ingested without removing these substances with smoked and burned appearance, substances such as amino acids, sugars and creatinine would be the main ingredients that react at more than 120 ºC to synthesize this mutagenic and carcinogenic cocktail.
Other industrially processed foods such as meat and smoked fish have concentrations of benzo[a]pyrene and other PAHs that can cause serious health damage. Organisms such as the Spanish Agency for Consumption, Food Safety and Nutrition
warn about the tolerable daily intake of PAH on occasion that they do not represent a risk to health. Many countries have established protective measures by means of advanced legislation on the maximum permissible limits in certain food products; hence the warning is made about the consumption of smoked products and those that are subjected to direct drying.

Polycyclic aromatic hydrocarbons are not only generated by natural sources and industry of hydrocarbons and their derivatives, also, are produced at home during the preparation of some foods that are subjected to cooking with charcoal or firewood at high temperatures; likewise, with exposure that produces smoking and burning.



Benzo[a]pyrene is highly carcinogenic and is present in cigarette smoke, foods subjected to high temperatures for preparation and other nearby sources.
PAHs have rapid effects such as eye irritation, skin and digestive symptoms; however, their greatest damage to health is manifested in the long term by mutagenic and carcinogenic disorders.
The cooking of foods rich in proteins and fats with firewood, charcoal or frying should be done avoiding the burning and generation of smoke due to excessive exposure.

- Polycyclic Aromatic Hydrocarbons (PAHs) Link
- Polycyclic aromatic hydrocarbon Link
- AECOSAN - Agencia española de consumo, seguridad alimentaria y nutrición Link
- Agudo, A. (2009). Los Hidrocarburos Aromáticos Policíclicos (HAP) Acercamiento a su problemática como riesgo laboral Link

For the elaboration of the images in gif format, the software ChemOffice 2016, Chemsketch, Easy GIF Animator and photoshop cs6 were used.
For the elaboration of the images in gif format, the software ChemOffice 2016, Chemsketch, Easy GIF Animator and photoshop cs6 were used.
𝕿𝖍𝖆𝖓𝖐 𝖞𝖔𝖚 𝖋𝖔𝖗 𝖞𝖔𝖚𝖗 𝖘𝖚𝖕𝖕𝖔𝖗𝖙 @𝖚𝖕𝖒𝖊𝖜𝖍𝖆𝖑𝖊, @𝖘𝖙𝖊𝖊𝖒𝖘𝖙𝖊𝖒, @𝕿𝖍𝖊𝖗𝖎𝖘𝖎𝖓𝖌 @𝖊𝖈𝖔𝖙𝖗𝖆𝖎𝖓, @𝖈𝖚𝖗𝖆𝖓𝖌𝖊𝖑...

#stem #naturalproducts #ecotrain #oc #creativecoin #lifestyle #neoxian #health #palnet #naturalmedicine
Interesting that this occurs not just with chargrilled or BBQd meat, but also vegetables. It's common here in Thailand to blacken eggplants on hot coals, and then use that to make chili. Really interesting, relevant and useful material. Thank you!
Leading the curation trail for both @ecotrain & @eco-alex.
Together We’re Making This World A Better Place.
Click Here To Join the manually curated trail "@artemislives" to support quality eco-green content.
Hi @artemislives. Thank you very much for reading and appreciating this publication. With your comment you complement this information. In truth, sometimes we believe that preparing food on direct and intense fire is something beneficial; however, it is very harmful to the organism.
First time that I use the label #ecotrain; I did it by reference of my husband @ulisesfl17 who has used it to share part of his publications according to the themes.
I loved your comment. A hug
A hug back to you @arac Welcome to use the #ecotrain tag for things that speak to eco-community and the environment. Preparing food well is something we could ALL learn a lot more about.
Thanks for sharing this amazing post!
Thank you for visiting this publication and commenting on its valuable content.
Happy day
Thank you for visiting this publication and commenting on its valuable content.
Happy day
Thanks for sharing with us on NaturalProducts.Today.
Thanks for the support. Happy day
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app. This granted you a stronger support from SteemSTEM. Note that including @steemstem in the list of beneficiaries of this post could have yielded an even more important support.
Thanks for the support. Happy day
Congratulations @arac! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Thank you for recognizing my work. Happy day