[Lets learn Physics #2] Understanding first law of thermodynamics and its applications..
Understanding first law of Thermodynamics
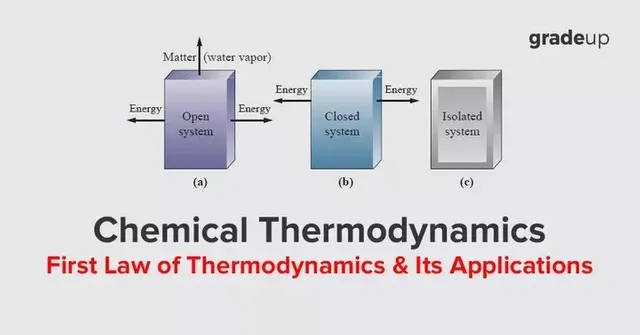
Introduction to first law of thermodynamics
First law of thermodynamics is basically the law of conservation of energy. According to which energy can neither be created nor be destroyed but can be change from one form to another form.
According to first law of thermodynamics, if a heat (Q) is applied to system, a part of it converts into work(W) and another part of it is used to increase internal energy(dU) of the system.
Mathematically we can write:-
For a gaseous system, if P is the pressure and dV is change in volume, then work done is given by :-
Therefore, equation (1) becomes :-
What is Q, dU and W
Heat Energy(Q):-Heat energy simply amount of thermal energy or heat which is transferred from one system to another system which are at different temperatures, when come in contact with each other.
For Example:- See the image below, in which heat is transferred from surrounding to ice, causing melting of ice..

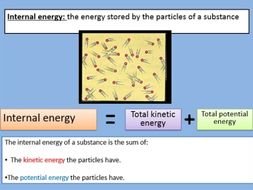
Internal Energy(U):- Internal energy is simply the energy associated to system due to its kinetic energy at motion and potential energy at rest. As kinetic energy of the system increase with increase in temperature, so internal energy also increases with increase in temperature and vice - versa.
Work done (W):-Work done is simply the measure of quantity by which given system expanded or contacted.
Sign convention for work done and heat for first law

Kindly note that work done that we have taken in equation (1) and (2) is work done on the system and hence negative, we can use work done by the system which can taken as positive.
History of first law of thermodynamics
The need for study of nature of heat and work done and their relationship began with the invention of engines which were used to extract water from mines.The first full statement of law was given by Rudolf Clausis and William Rankie in 1850. Although the William Rankie's statement of first law of thermodynamics is not considered
Rudolf in 1850, gave the statement of first law of thermodynamics for cyclic process as follows:-
In all the cases in which work is produced by the agency of heat,a quantity of heat is consumed which is proportional to work done ; and conversely, by the expenditure of an equal quantity of work an equal quantity of heat is produced.
Evidences in support of first law of thermodynamics
There are large number of evidences in support of first law of thermodynamics. Here we will discuss examples from our daily life.
We know first law simply states that the change in internal energy of a system is equal to total heat supplied to the system minus work done from the system.
i.e.
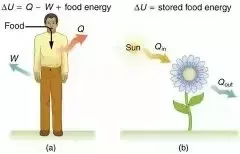.webp)
The above diagram (a) is showing that when we take food, which ultimately gets converted into energy. Now some part of this energy is used to increase internal energy i.e. motion inside human body and rest of it is used as work, that we do in our daily life.
Similarly, diagram(b) is showing that when a flower takes heat energy from sun, a part of it is used to prepare food i.e. for work done and rest of it is used to store food in form of internal energy.
Significance of first law of thermodynamics
First law of thermodynamics help us to understand the nature of various thermodynamic processes, when heat is supplied to them.Let study it one by one.
- Significance on isothermal process:- The thermodynamic process that takes place at constant temperature is called isothermal process.
Here Temperature= Constant.
As temperature of system is directly proportional to internal energy of system,so we can say that:-
Internal Energy = constant, so
Using dU=0 in first law of thermodynamics we get:-
This means that if any system operates under isothermal process, the heat supplied to it will only use for work done.
significance on adiabatic process:- A thermodynamic process in which there is no flow of heat from system to surrounding or surrounding to system is called adiabatic process.
So here ,
Q = 0
Using Q = 0 in first law of thermodynamics we get:-
W = -dU
This means that if work is done by the system, its internal energy decreases and if work is done on system, its internal energy increases.
- Significance for isobaric process:- * A process which operates at constant pressure, is called as isobaric process*.
For isobaric process,
Using **P = constant ** in first law of thermodynamics, we get:-
This means that for a isobaric process, heat supplied is used to increase internal energy as well as to do pressure-volume work.
- Significance for isochoric process:- A thermodynamic process, which operates at constant volume is called as isochoric process
i.e. for isochoric process Volume = constant
So,
Using dV = 0 in first law of thermodynamics we get;-
This means that if a system operates under isochoric process, the heat supplied to it is used only for increase its internal energy.
By using all these results we can say that :-
Energy of isolated system is constant i.e. energy of whole universe is constant.
For work done and to increase internal energy, we require certain amount of heat. There cannot be any device which can give output as work without taking any heat as input. Such devices are called perpetual motion machine of first kind , which is not possible.
Applications of first law of thermodynamics
There are numerous applications of first law of thermodynamics in our day to day life.For example:-
The light bulbs, electric fans and all other appliances at our home are based upon first law of thermodynamics,because they transform electrical energy into light energy, mechanical energy and also to some other kinds of energies.
Plants which are major source of food for living beings, make their own food by converting light energy from Sun into chemical energy.
Our body too converts food into chemical and mechanical energy.
There are also large number of devices which work on the principle of first law of thermodynamics, some of them are:-
Turbines, boilers, heat exchanger , refrigerators, rockets and bomb explosion etc.
Citations:-
(https://gradeup.co/first-law-of-thermodynamics-and-its-applications-i-2050908c-8b89-11e6-9433-f44d53741b5d)( only image no.1 is used)
*(https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/internal-energy-sal/a/heat) (second number image is used from here)
(https://www.tes.com/teaching-resource/internal-energy-differentiated-lesson-for-trilogy-aqa-combined-science-11568874)(third image is used from here )
(https://www.chegg.com/homework-help/questions-and-answers/figure-shows-container-sealed-top-amovable-piston-inside-container-ideal-gas-at100-200-and-q818070)(fourth image is used from here)
(https://www.slideshare.net/jonialbarico/the-laws-of-thermodynamics)(fifth image is used from here)
(https://www.quora.com/What-is-the-proof-of-first-law-of-thermodynamics)(sixth image is used fro here)
(https://www.quora.com/What-are-the-significance-of-first-law-of-thermodynamics)
(https://www.quora.com/What-are-the-applications-of-the-first-law-of-thermodynamics)
Thanks for reading.