A cure for ALL
I know that it would be cool to have a universal cure for all diseases, but unfortunately, that will remain a fantasy. In this post I am going to talk about something real: Acute Lymphoblastic Leukemia (ALL). This is a type of cancer that is really deadly, and the brutal part is that in many instances it affects also children. ALL is a cancer that involves the bone marrow and, as a consequence, also the blood.
Usually our bone marrow produces immature cells that will then differentiate and populate our blood, some cells will become B lymphocytes, some other red blood cells, T cells, platelets etc..please note that for clarity-sake I’m over simplifying.
Basically, in children with ALL, their bone marrow produces too many B-cells that also don’t work properly. So they can’t fight infection properly and by being so abundant in the blood stream, they can also affect the generation of other healthy cell types in the blood. As a consequence, the whole homeostasis in the bodies of these patients is compromised. They suffer of recurrent infections, anemia, bleeding etc..if not treated on time this cancer will be fatal.
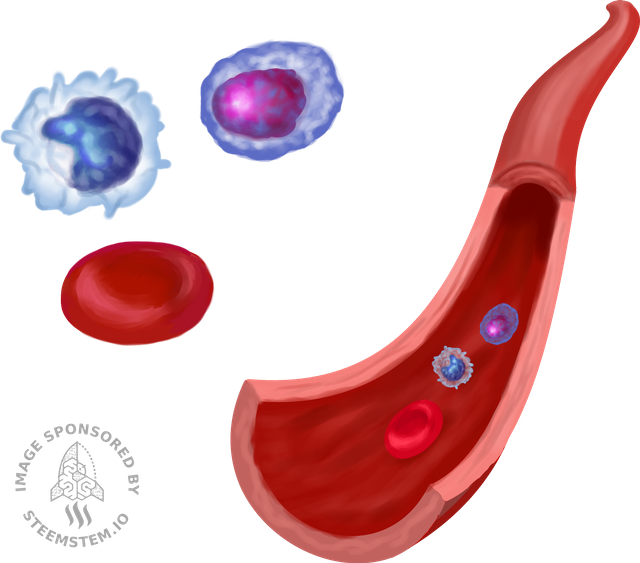
Image created by @pab.ink

Image created by @pab.ink
Back in the 70s the survival rate was only 31% but progress in the field allowed us to increase the survival rate up to 90% (Ward et al. 2014). Although this is reassuring, there are also bad news. 2-3% of all the patients that received treatment will not respond well (Schrappe et al. 2012) and 10-15% will relapse, which means that the cancer will come back (Hunger and Mullighan 2015).
In general, ALL is the predominant cause of cancer-related deaths in children (Ko et al. 2010) and the saddest part is that children that already suffer from other diseases are particularly vulnerable, for example kids with down syndrome are more likely to suffer relapse and in such event, their survival rate is only 10-25% (Hitzler et al. 2014).
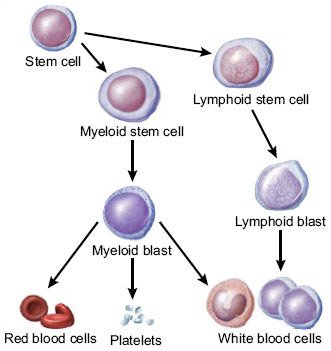
Ibdipcan2015 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons

Ibdipcan2015 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons
So, how do we fight leukemia?
The main strategy consists in chemotherapy, with the administration of high doses of drugs (Raetz et al. 2008). Unfortunately, this alone is not sufficient to sustain cancer remission over the long term (Hucks and Rheingold 2019). Some patients may qualify for hematopoietic stem cell transplants (SCT) to help their bone marrow generate healthy cells (Eapen et al. 2008). Unfortunately, even after receiving SCT, the 29% of the patients still relapsed (Crotta et al. 2017). After this relapse, only 33% of the patients will survive over a 3 years period (Bondarenko et al. 2016).
Luckily, other strategies such as immunotherapies showed really promising results. Most immunotherapies rely on the fact that the cancer B cells express a specific surface marker which is called CD19. This enables us to generate drugs that can target specifically the cancer B-cells, increasing the efficacy with fewer side-effects. An example is a drug called Blinatumomab. This is a monoclonal antibody that can form a bridge between T cells and the B-cells that express CD19, allowing the destruction of the B-cells. When this drug was tested on adult patients it showed the 43% response rate (Klinger et al. 2012). But, the efficacy was slightly lower in children, with 39% (von Stackelberg et al. 2016).
CCO Creative Commons
CCO Creative Commons
CAR-T may be the solution
A newer approach consists in harvesting the patient’s T cells and educate them to recognize and destroy the cancer B-cells that are CD19 positive. Basically, the patient’s T cells are infected with a virus that allows them to express the chimeric antigen receptor (CAR), a receptor that can target CD19, so from here the name CAR-T (Hucks and Rheingold 2019). Initial clinical studies revealed that CAR-T is really effective, in fact 80 to 90% of the patients had remission of ALL (Maude et al. 2018) and this lead the first CAR-T based drug to be approved by the FDA in 2017, the name of the drug is Tisagenlecleucel. Harnessing the patient’s own immune system to deal with the cancer is an elegant way to solve the problem and it’s an approach that can be translated into clinical use. CAR-T allows to avoid chemoresistance, has fewer side effects and it’s very specific, it targets only cancer cells (Hucks and Rheingold 2019).However, CAR-T has also some side effects. For instance, a few days after the infusion of CAR-T cells, doctors observed neurological toxicities that caused encephalopathy, confusion, delirium, tremor agitation and somnolence. Luckily, these symptoms are just transient and they are over after a few weeks (Maude et al. 2018). Another issue is the fact that these virally modified cells stay in the patient for quite some time, a study found that CAR-T treated patients still had virally modified cells in their systems after 2 years (Maude et al. 2014). A consequence of CAR-T therapy is also B-cell aplasia (depletion of B cells), which makes the patient more likely to develop infections (Hucks and Rheingold 2019).
This is not the end of the story. There are still some challenges to overcome. For example, the CAR-T therapy selected in some patients cancer B cells that are CD19 negative, thus these cancer cells can easily elude CAR-T. A solution is to engineer Bivalent CAR-T cells that are specific for 2 receptors: CD19 and CD22, this strategy is currently being investigated (Fry et al. 2018). The last remark I would like to make is that we need more alternatives to viral vectors to engineer CAR-T cells, that would increase considerably the safety of the therapy.
References
Bondarenko, Sergey N. et al. 2016. “Allogeneic Hematopoietic Stem Cell Transplantation in Children and Adults with Acute Lymphoblastic Leukemia.” Cellular Therapy and Transplantation 5(2): 12–20. http://cttjournal.com/en/archive/vypusk-5-nomer-2/regulyarnye-stati/allogennaya-transplantatsiya-gemopoeticheskikh-stvolovykh-kletok-u-detey-i-vzroslykh-s-ostrym-limfob/.
Crotta, Alessandro et al. 2017. “Disease Characteristics and Overall Survival in Pediatric Patients with Relapsed and Refractory B-Cell Acute Lymphoblastic Leukemia after Stem Cell Transplantation.” Biology of Blood and Marrow Transplantation 23(3): S50. https://linkinghub.elsevier.com/retrieve/pii/S1083879116305420.
Eapen, M et al. 2008. “Outcomes after HLA-Matched Sibling Transplantation or Chemotherapy in Children with Acute Lymphoblastic Leukemia in a Second Remission after an Isolated Central Nervous System Relapse: A Collaborative Study of the Children’s Oncology Group and the Center.” Leukemia 22(2): 281–86. http://www.ncbi.nlm.nih.gov/pubmed/18033318.
Fry, Terry J et al. 2018. “CD22-Targeted CAR T Cells Induce Remission in B-ALL That Is Naive or Resistant to CD19-Targeted CAR Immunotherapy.” Nature medicine 24(1): 20–28. http://www.ncbi.nlm.nih.gov/pubmed/29155426.
Hitzler, Johann K et al. 2014. “Outcome of Transplantation for Acute Lymphoblastic Leukemia in Children with Down Syndrome.” Pediatric blood & cancer 61(6): 1126–28. http://www.ncbi.nlm.nih.gov/pubmed/24391118.
Hucks, George, and Susan R. Rheingold. 2019. “The Journey to CAR T Cell Therapy: The Pediatric and Young Adult Experience with Relapsed or Refractory B-ALL.” Blood Cancer Journal 9(2): 10. http://www.nature.com/articles/s41408-018-0164-6.
Hunger, Stephen P, and Charles G Mullighan. 2015. “Acute Lymphoblastic Leukemia in Children.” The New England journal of medicine 373(16): 1541–52. http://www.ncbi.nlm.nih.gov/pubmed/26465987.
Klinger, Matthias et al. 2012. “Immunopharmacologic Response of Patients with B-Lineage Acute Lymphoblastic Leukemia to Continuous Infusion of T Cell-Engaging CD19/CD3-Bispecific BiTE Antibody Blinatumomab.” Blood 119(26): 6226–33. http://www.ncbi.nlm.nih.gov/pubmed/22592608.
Ko, Richard H et al. 2010. “Outcome of Patients Treated for Relapsed or Refractory Acute Lymphoblastic Leukemia: A Therapeutic Advances in Childhood Leukemia Consortium Study.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28(4): 648–54. http://www.ncbi.nlm.nih.gov/pubmed/19841326.
Maude, Shannon L et al. 2014. “Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia.” The New England journal of medicine 371(16): 1507–17. http://www.ncbi.nlm.nih.gov/pubmed/25317870.
Maude, Shannon L et al. 2018. “Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia.” The New England journal of medicine 378(5): 439–48. http://www.ncbi.nlm.nih.gov/pubmed/29385370.
Raetz, Elizabeth A et al. 2008. “Chemoimmunotherapy Reinduction with Epratuzumab in Children with Acute Lymphoblastic Leukemia in Marrow Relapse: A Children’s Oncology Group Pilot Study.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology 26(22): 3756–62. http://www.ncbi.nlm.nih.gov/pubmed/18669463.
Schrappe, Martin et al. 2012. “Outcomes after Induction Failure in Childhood Acute Lymphoblastic Leukemia.” The New England journal of medicine 366(15): 1371–81. http://www.ncbi.nlm.nih.gov/pubmed/22494120.
von Stackelberg, Arend et al. 2016. “Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology 34(36): 4381–89. http://www.ncbi.nlm.nih.gov/pubmed/27998223.
Ward, Elizabeth et al. 2014. “Childhood and Adolescent Cancer Statistics, 2014.” CA: a cancer journal for clinicians 64(2): 83–103. http://www.ncbi.nlm.nih.gov/pubmed/24488779.
Communities that support me are:

Image created by @gtg

IMMAGINE CC0 CREATIVE COMMONS, si ringrazia @mrazura per il logo ITASTEM. Click here and vote for @davinci.witness
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thank you for your research. Also, 80 to 90% remission is fantastic. Question, does the rate of B-Cell aplasia decrease over time as the CAR-T cells diminish? Or are the patients stuck with a new disorder?
it the number of CAR-T decreases the number of B cells should go up again..
So it's basically like infecting the patient with an autoimmune disease that targets only cancer cells, very clever. Genetic engineering might help with this goal, by further modifying the virus used and eliminating the negative side effects.
Very ingenious title btw, really got my attention lol!
Thanks :)
Educating cells to recognize and fight diseases. Sounds like a winner...
Quite a lot of side effects and challenges there. i do hope they can overcome them in the future!
Sounds promising for sure!
$rewarding 100% 14min
The reward of this comment goes 100 % to the author aboutcoolscience. This is done by setting the beneficiaries of this comment to 100 %.
:D
Thank you :)
You got me with the 'ALL' title. I was initially expecting something else.
Just a question. when you mention viral car-T cell are still present after two years, I imaging this is in a rate that is way higher than expected. How large is this rage (and also, how would you compare it with something a physicist like me could understand)? and is this really a problem?
ahahah sorry about that, I just could not resist. In my defence some publications about the disease also use similar allusions about the word "ALL". You always have smart questions.. even if today we don't know if virally engineered cells can cause health issues over the course of several years, they for sure create uncertainty and it may make it more difficult to gain FDA approval. But virus are not the only tool at our disposal, several people prefer using physics to engineer cells with electroporation. It's a bit of a conundrum, you want your CAR-T to stay in circulation for long enough to maintain cancer remission but not too long. The fact that in some patients they observed neural toxicity and depletion also of healthy B cells could create some concerns, yet it's better than being dead.
I am pretty convinced I would have been as weak as you in your case, and made the joke as well :) So don't worry!
Along both research lines, it is good to see the progresses. Cancer is still one of the major causes of death in humans. Thanks for the answer!
Hi @aboutcoolscience!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation!
Your UA account score is currently 4.563 which ranks you at #1840 across all Steem accounts.
Your rank has improved 3 places in the last three days (old rank 1843).
In our last Algorithmic Curation Round, consisting of 380 contributions, your post is ranked at #45.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server
Hi @aboutcoolscience!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @aboutcoolscience!
You raised your level and are now a Dolphin!
@aboutcoolscience You have received a 100% upvote from @steemconductor because this post did not use any bidbots and you have not used bidbots in the last 30 days!
Upvoting this comment will help keep this service running.