Basic aspects of thermodynamics. Part I
Introduction
The careful study of some concepts is essential to achieve a good understanding of the topics dealt with by thermodynamics.
What is thermodynamics?
Thermodynamics can be defined as the science of energy. Although everyone has an idea of what energy is, it is difficult to define it precisely. Energy can be considered as the ability to cause changes. The term thermodynamics comes from the Greek words therme (heat) and dynamis (force), which corresponds to the most descriptive of the first efforts to convert heat into energy.[Textual quotation]: Chapter: Introduction and basic concepts. page: 2. Book of thermodynamics. Authors: YUNUS A. ÇENGEL and MICHAEL A. BOLES. 7th edition.
It is important to mention that energy is a form of expression of the universe in different forms, magnitudes and dimensions, so we think that thermodynamics is only responsible for the dynamism in the transfer of heat, which is also a form of energy, but that time is not the only way, as we all know there is kinetic energy, potential energy, hydraulic energy, electrical energy, mechanical energy among others. In each of these forms of energy, and in its transformations, there are losses or gains of heat, that is to say changes in temperature, but it is not only to these changes that its study is limited, thermodynamics becomes greater when it takes diversity of the broad study in the ways of manifesting energy in various industrial processes where engineering and science are the protagonists.
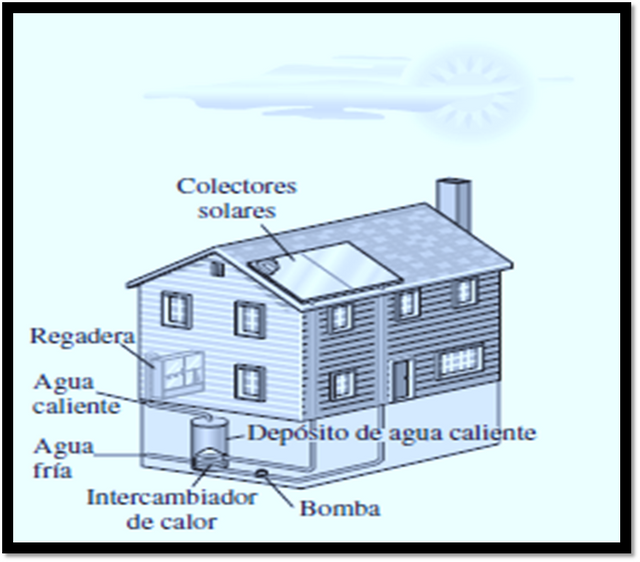 [Image 1]
[Image 1]
Note: The design of many engineering systems, such as the one represented in Image 1, which has a solar system to heat water, has to do with thermodynamics. Image taken from Cengel thermodynamics book and edited at Microsoft Powerpoint.
Importance of dimensions and units in the study and understanding of thermodynamics
Any physical quantity is characterized by dimensions. The magnitudes assigned to the dimensions are called units. Some basic dimensions, such as mass m, length L, time t and temperature T are selected as primary or fundamental dimensions, while others such as velocity V, energy E and volume V are expressed in terms of the primary dimensions and are called secondary dimensions or derived dimensions. Over the years several systems of units have been created. Despite the great efforts that the scientific community and engineers have made to unify the world with a single system of units, two of these are still in common use today: the English system, which is known as United States Customary System (USCS) and the SI metric (from Le Système International d'Unités), also called the international system. The SI is a simple and logical system based on a decimal relationship between the different units, and is used for scientific and engineering work in most of the industrialized nations, including in England. However, the English system has no obvious systematic numerical base and several units of this system relate to each other quite arbitrarily (12 inches = 1 foot, 1 mile = 5280 feet, 4 quarts = 1 gallon, etc.), which makes learning confusing and difficult. The United States is the only industrialized country that has not yet adopted the metric system completely.[Textual quotation]: Chapter: Introduction and basic concepts. page: 3-4. Thermodynamics book. Authors: YUNUS A. ÇENGEL and MICHAEL A. BOLES. 7th edition.
It is evident the importance of dimensions, mass, length and time are the dimensions that make up almost the basis of the whole structure that gives life to the understanding of science based mainly on physical phenomena. Now, as the great contributions were given by the hand of great philosophers and scientists such as Albert Einstein, Isaac Newton, among others, other dimensions had to be developed that gave him answers to basic phenomena such as the change of position of an object with Regarding time, we can study the changes that occur in the matter when they reach an acceleration. For this it was necessary to postulate laws that are governed by mathematical equations that give rise to secondary dimensions, that is, they derive from the primary ones as mass, length and time.
For all these measures, two systems that are the ones we know today, which are the British or English system, and the international system of measurements (SI), were practically parallel. In my opinion, in a practical way, none of the two systems It can play down the importance, since the way to unify them has been sought in only one and for different reasons it has not been possible, reason why we have to consider a way of knowing the equivalences of those units between the two systems, especially due to the fact that in reality the two systems are used in parallel. A clear example of these two systems we have in my country (Venezuela), here the oil industry manages the two systems, such is the case of drilling fluids, that when they are going to be tested in laboratories, many of the instruments used use scales of the international system, once these data are obtained, the conversion to the English system is required, since the equations of the manuals that we inherit from the gringos are in English system measurements.
Closed and open systems
A system is defined as a quantity of matter or a region in the space chosen for analysis. The mass or region outside the system is known as surroundings. The real or imaginary surface that separates the system from its surroundings is called the border. The border is the contact surface shared by the system and surroundings. In mathematical terms, the border has zero thickness and, therefore, can not contain any mass or occupy a volume in space. Systems can be considered closed or open, depending on whether a fixed mass or a fixed volume in space is chosen for study. A closed system (also known as a control mass) consists of a fixed amount of mass and no other can cross its border. That is, no mass can enter or exit a closed system. But energy, in the form of heat or work, can cross the border; and the volume of a closed system does not have to be fixed. If, as a special case, even energy is prevented from crossing the border, then it is an isolated system. An open system, or a control volume, as it is usually called, is a region appropriately chosen in space. Generally encloses a device that has to do with mass flow, such as a compressor, turbine or nozzle. The flow through these devices is best studied if the region within the device is selected as the control volume. Both mass and energy can cross the border of a control volume.[Textual quote]: Cengel book on thermodynamics, 7th edition. page: 10
It is very important to define a set in space that encloses a certain amount of mass to be studied, in this way I conceive a thermodynamic system, now this system is in the power to exchange energy with other systems, exchange mass and energy, or not to exchange neither mass nor energy, hence the definition of systems: closed, open or isolated, to understand and understand this part of thermodynamic systems, in that same proportion we will understand many of the functions of useful tools in real life, such is the case of compressors, pumps, heat exchangers, among others.
Here are some examples of the named systems:
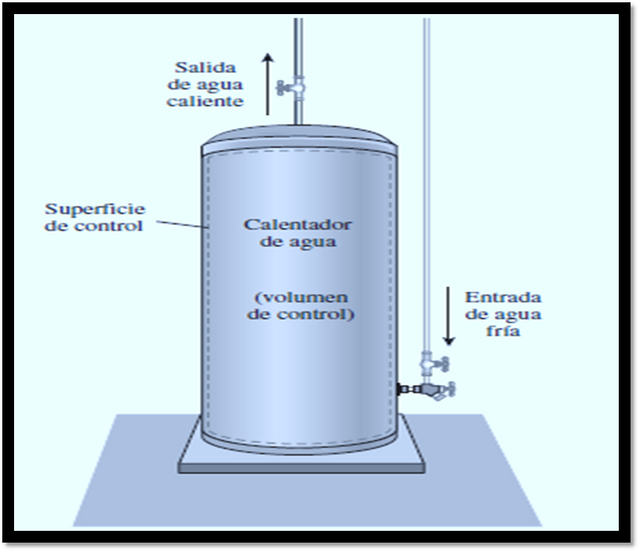 [Image 2]
[Image 2]
In Image 2, what is an open system is represented. Also called control volume, with one input and one output. Image taken from Cengel thermodynamic book and edited in Microsoft PowerPoint.
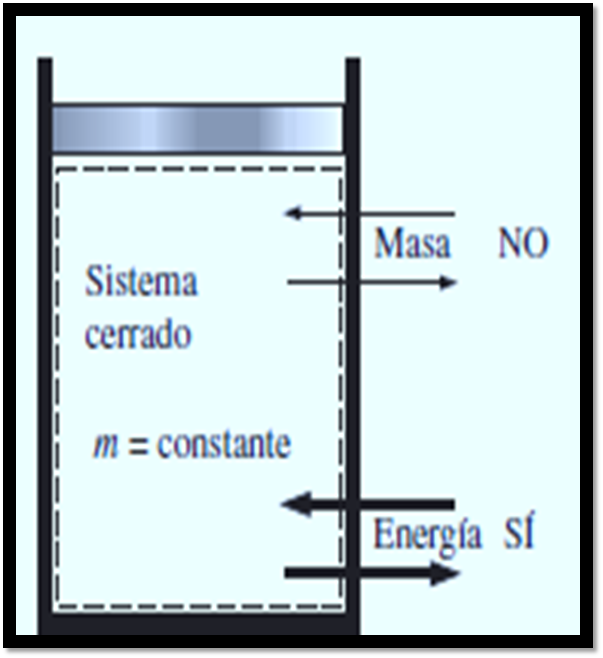 [Image 3]
[Image 3]
Figure 3 shows what would be a closed system, where there is energy exchange and not mass, in the piston-piston system the gas is trapped generating a heat that is transferred with the surroundings of the container, but nothing from the world the mass of gas contained in it is lost. The image was taken from the same book mentioned above and edited in Microsoft PowerPoint.
Considerations and Conclusions
There are many other aspects, which are basic and at the same time of importance for the study and understanding of thermodynamics, in this publication I only wanted to interpret 3 basic aspects:
- Concept of thermodynamics.
In this concept it is very important to understand the scope and limitations of the study of thermodynamics.
- The importance of measurement and conversion systems within thermodynamics.
Like the physics, chemistry and other branches derived from these it is important to understand the systems of measurements, since we measure the energy, be it in joule, in BTU, likewise the temperature as degrees Celsius, degrees Fahrenheit among others, keeping in mind the two systems, the British and the international system, it is very important to know how to carry out the transformation processes of the units in the two systems.
- System concept. Open system, closed and isolated.
To keep clear the law of conservation of energy, we need to understand that what enters is equal to what comes out, that is why what enters a system is equal to what comes out, these systems do share mass and energy, can be considered isolated, closed or open. The important thing about this case is to be able to compare each of the cases with some machines that are used today.
To explain other important basic aspects of thermodynamics, I will use a second and third part, to complement what is explained in this article.
Greetings and I hope this descriptive material is useful.
Bibliography consulted.
Thermodynamics book. YUNUS A. ÇENGEL. MICHAEL A. BOLES. Seventh edition. Editorial Mc Graw Hill. Mexico, D.F.
Congratulations @haf67! You have completed the following achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
SteemitBoard and the Veterans on Steemit - The First Community Badge.