Carbon gels and synthesis
Carbon Gels
1. Description.
Carbon gels are porous materials with a formed lattice structure by interconnected particles composed mainly of carbon, of form Spheroidal and nanometric size. These materials are obtained by carbonization of organic gels derived from the polycondensation of hydroxylated benzenes (resorcinol, phenol, catechol, hydroquinone, etc.) and aldehydes (formaldehyde, furfural, etc.). Actually, they are not gels themselves, since a gel is composed of a solid phase and a liquid phase. However, post that is obtained from the drying and carbonization of organic gels, it has been maintained the gel nomenclature for the carbonaceous material.
The porosity of these materials is located both inside the particles which make up the material, characterized by micropores, such as among the particles, which they give rise to mesopores and macropores.
This type of nanostructure is responsible for some unique properties that allow the application of these materials in many fields, such as supercapacitors, adsorbents, thermal and acoustic insulators, support catalysts for different processes, and catalytic devices such as fuel cells.
The most remarkable characteristic of this type of materials lies in the possibility to adjust its textural properties during the preparation method to satisfy the desired application. In addition, carbon gels have the advantage that they can get in various forms: monoliths, dust, films, microspheres, etc., which favors your use in very different fields.
2. Synthesis
The synthesis of carbon gels is generally carried out in four stages: the first stage takes place the formation of the organic gel by polycondensation of the reagents; in the second stage the aggregates that form the gel cross over to forming the porous reticular structure, in a process that is usually called curated; in the The third stage is followed by drying and, in a final stage, the pyrolysis of the organic gel, which will give rise to the carbonaceous material and which we will call gel carbon.
2.1. First stage: addition reaction.
Resorcinol and formaldehyde are usually used as reagents precursors of the gel. The resorcinol molecule is a benzene ring with two hydroxyl groups at positions 1 and 3, which induce an electro-donor effect in the ortho- and para- positions of the benzene ring, which allows the addition of formaldehyde (by aromatic electrophilic substitution) in positions 2, 4 and 6 of the ring.
For the addition of formaldehyde to occur, one of the -OH groups of The resorcinol molecule must be deprotonated, for which bases such as Na2CO3, NaOH, etc (which we will call basifying agents). So in the first stage, called addition, the activated resorcinol ring (resorcinol anion) adds formaldehyde, forming hydroxymethyl groups (-CH2OH).
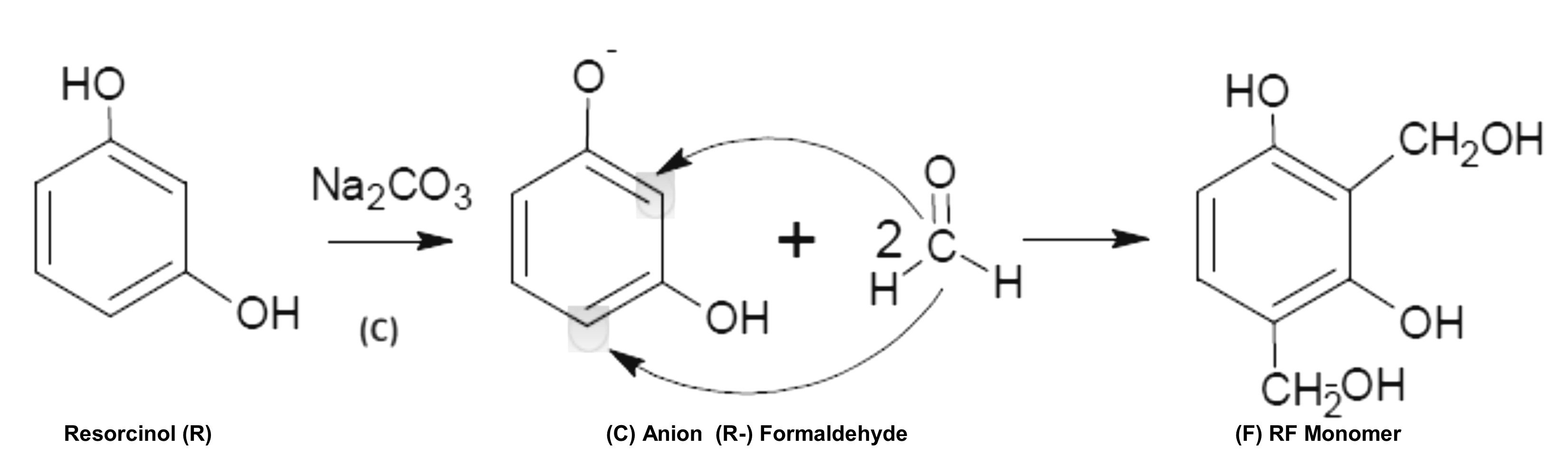
Polymerization mechanism between the resorcinol and formaldehyde molecules:
Addition reaction.
2.2. Second stage: condensation reaction.
In a second stage, called condensation, groups hydroxymethyl condensates forming methylene groups (-CH2-) and methylene-ether bridges (-CH2OCH2-) in the form of aggregates. These aggregates form particles Colloidal crosslinks with diameters between 3 and 20 nm. The size and number of the aggregates generated during the polymerization depend on the ratio resorcinol / basifying agent.
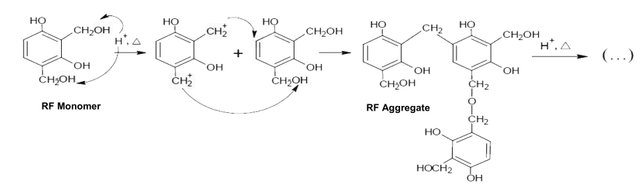
Polymerization mechanism between the resorcinol and formaldehyde molecules:
Condensation reaction.
After this stage, the polymer particles begin to aggregate in an interconnected structure that resembles a coral and that occupies the original volume of the dissolution.
The addition of a basic agent (called a catalyst in the literature) is important for the initial formation of resorcinol anions in the addition reaction, thereby activating the benzene ring to form the group hydroxymethyl, which is essential for the following condensation reactions. Strictly speaking, sodium carbonate (usually employed as a basic catalyst), is not a catalyst itself. Its main role is that of increasing the pH of the aqueous solution of resorcinol-formaldehyde, and therefore, favor the deprotonation of the benzene ring. It has been shown that the relationship
Resorcinol / basifying agent influences the final properties of the gel, that is, a greater or lesser amount of basifying agent, and therefore, a pH more or less basic, will give rise to gels with different physical-chemical properties.
2.3. Third stage: drying.
Occasionally, the gels are subjected to a pre-drying treatment that consists of the replacement of the solvent (water), remaining in the gel after curing, by another solvent of a different nature (organic), such as acetone, methanol, isopropanol, etc. The replacement of the solvent partially prevents the collapse of the Porous structure of the gel during drying, due to surface tensions generated by the evaporation of the solvent. The solvents employees as substitutes have a lower surface tension than water, and so, therefore, the tensions generated in the pores when evaporating are lower (γAgua (20 ºC) = 72.75 · 10-3 N · m-1; γ Acetone (20 ºC) = 23.7 · 10-3 N · m-1), allowing the original porous structure to be conserved better.
Subsequently, the drying of the organic gel is carried out. The choice of one u Another method of drying will lead to different types of materials, aerogels (drying super-critical), xerogels (subcritical drying, either by evaporation, or convection) and cryogels (cryogenic drying).
2.4. Fourth stage: pyrolysis.
Finally, the organic gel once dry, is subjected to a pyrolysis in atmosphere inert at high temperature (usually between 700-1050ºC) to obtain the material carbonaceous The heating rate must be small to avoid large tensions in the gel skeleton, due to the exit of gases from the inside from the pyrolysis of organic precursors (due to breakage of the C-O and C-H bonds). The loss of mass during pyrolysis is usually around 50% depending on the temperature. The pyrolysis process increases the number of micropores, which leads to an increase in the surface area of the carbon gel.
References: