Glass - Solid or liquid?
Glass is a strange state of matter. It is sometimes heard that it is an extremely viscous liquid, but it is a little more than that.
To understand a part of his secret, one must not only look at his physical properties, but also the way in which he is made.
As we learn at school, there are essentially three states of matter: solids, liquids and gases. The distinctive feature of solids is that they are crystals: when viewed at the atomic scale, they reveal an ordered structure, where the atoms are arranged in a crystal lattice.

The image shows the crystal structure of quartz (SiO2). Each silicon atom (in blue) is bonded to it with 4 oxygen atoms (in red), in a periodic pattern.
The ordered crystal structure of solids contrasts with the situation of liquids and gases, which are disordered states. At the microscopic level, the molecules that compose them are distributed randomly, and for good reason they keep moving!
![LVJX3577[1].jpg](https://steemitimages.com/DQmQg4dDBEUz6sC7ZnhL1fvuGdnFBZaRhFJQDjuajyFrftJ/LVJX3577%5B1%5D.jpg)
The glass structure
The glass of our panes and our bottles is a solid material, so we can think that it also has a crystalline structure. But if you look at it on a microscopic scale, it is not! The atoms that compose it are distributed in a totally disordered manner, as in a liquid. And yet these atoms are well fixed and motionless, as in a solid. The glass is said to be an amorphous solid.
You know, we get glass by melting sand. Now the sand consists essentially of quartz, which, as we have seen above, is a crystalline solid. Is not that contradictory?
How can the glass be amorphous, while it is obtained from sand that is crystalline?
The answer lies in a phenomenon supercooling. By heating and cooling the quartz which composes the sand, it can be brought to remain in a liquid state without the transition to the crystal being made. Glass is a supercooled liquid!
A conventional glass composition (which does not include only sand) has a theoretical crystallization temperature around 1000 ° C. But we make sure that this crystallization never starts. To understand this state of supercooling (a little different from that of water), one must be interested in the viscosity of molten glass.
![MFHD6055[1].jpg](https://steemitimages.com/640x0/https://steemitimages.com/DQmTMVouNa2FJbvimEYR6iHvSz5cpz4sdE6mFduyibcVViK/MFHD6055%5B1%5D.jpg)
The viscosity of the glass
Viscosity, which we all have a more or less intuitive idea. The viscosity of the liquids generally depends on the temperature. For molten glass as for many liquids, the higher its temperature, the more fluid it is.
The figure shows the viscosity of a conventional glass as a function of its temperature. As you can see, the change in viscosity is so violent that it must be represented on a logarithmic scale.
At 1500 ° C., the glass has a viscosity close to that of honey (10 Pa.s, ie 10,000 times the viscosity of the water), but when it is cooled, it increases very rapidly. So fast that at a temperature of 500 ° C, glass is about 10 ^ 19 times more viscous than water!
The manufacture of glass: the search for supercooling
Let us see how in practice one reaches the state of supercooling necessary to obtain the glass. We start with sand (and some other ingredients) which are heated to about 1500 ° C. Suppose the mixture is cooled at a substantially constant rate, and we follow what happens over time.
The graph below shows the evolution over time of the temperature (in blue), of the viscosity (in red), and of a third quantity: the crystallization rate of the mixture.
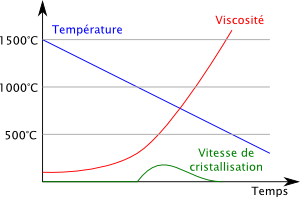
Between 1500 ° C. and 1000 ° C., the mixture cools, its viscosity increases, and since we are above the crystallization temperature (1000 ° C.), the crystallization rate is zero.
When the temperature reaches 1000 ° C., the crystallization can in principle start, but starts at a low speed. The more our glass is cooled to below 1000 ° C, the higher its crystallization rate. But rather rapidly the increase in viscosity is such that it prevents crystallization, and the rate of crystallization falls to zero.
At about 550 ° C., the viscosity is such that the molecules are completely immobile. The glass has become an amorphous solid and is said to have passed the glass transition temperature.
In a true glass manufacturing process, if the temperature of the glass is reduced sufficiently quickly, the crystallization does not even have time to start and is suffocated by the viscosity. That's why glass is a supercooled liquid.
It is not only with conventional glass that an amorphous solid can be obtained by very fast cooling of a supercooled liquid. With such processes, it is possible to obtain metallic glasses, and even glasses of water (ie, amorphous solid water).
![UTHX4384[1].jpg](https://steemitimages.com/DQmSQMZPQfDPHiSNBEoqwSSCrcNJuzPqfG4WT5m7ysWdvnG/UTHX4384%5B1%5D.jpg)
Glass at the bottom of the windows?
Finally, and for the most physicists among you, let us attack the idea that we sometimes hear when we speak of glass as a very viscous liquid: the proof would be that at the bottom of certain ancient glazings, Finds that the glass is thicker, as if it had sunk.
Let's do some dimensional analysis to see if it makes sense. The viscosity is expressed in Pa.s. It is therefore a pressure multiplied by a time. Let us calculate the pressure which a window undergoes under its own weight, and deduce in a time characteristic for the flow.
Suppose a glass of height h, section S and density \ rho. The weight of the pane is therefore F = \ rho g h S. Since it is applied to surface S, the pressure of the pane on itself is P = F / S = \ rho g h. For a glass of 1 meter high with a density of about 2500 kg / m3, it makes a pressure of the order of 10 ^ 4 Pascals.
By dimensional analysis, a time is calculated from a pressure P and a viscosity \ eta by making \ tau = \ eta / P. Let us apply numerically: even for glass at 500 ° C (viscosity 10 ^ 16), it would take 10 ^ 12 seconds for it to flow under the effect of its own weight. It's been a few tens of thousands of years! And I only took the viscosity at 500 ° C ...

Interesting. The melting pane of glass often crosses my mind. I'm by no stretch of the imagination a scientist or anything but certain things like this capture my imagination. I know this seems random, but sometimes i look at trees and consider how they grow and seem to "flow" around and over objects. They remind me of how lava flows and then cools and hardens. Just a thing I think about :)
Hmmm. you have a creative mind. thank you for stopping by
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Mr. Dark from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, and someguy123. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you like what we're doing please upvote this comment so we can continue to build the community account that's supporting all members.
Upvoted & RESTEEMED :]
Hey bro I followed u, pls follow back,
Varun from OT family