Dalton's law // Analysis

John Dalton(1766-1844), source image of mastery of wikimedia commons
Dalton's Law
This law enunciated by the chemist John Dalton, says to us that the quantities of the same elements, which react with a fixed quantity of other elements, to form different compounds are in the relation of entire simple numbers. In this effect, it is possible to consider to be two compounds, which form the carbon and the oxygen, in the carbon monoxide, CO, 1.33 is combined gr of oxygen with 1 gr of carbon, whereas in the carbon dioxide CO2, it gets together 2.66gr of oxygen with 1 gr of carbon, to compare the masses of oxygen, which combine with a fixed mass of carbon 1 gr, there is obtained a relation of entire simple numbers 1.33/2.66 = ½; ' The sum of the partial pressures of every gas, it is equal to the entire pressure of the gas miscellany '.
When any body exists in the elastic (gaseous) state, his fundamental particles are at a distance major than in any other state; every particle occupies the center of a relatively big sphere and supports his position supporting the others at a suitable distance, which would tend to diminish by the force of gravity or another reason.
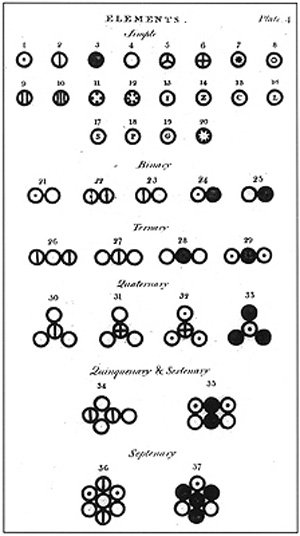
Several atoms and molecules represented in the book of titled Dalton A New System of Chemical Philosophy (1808)., source of image of mastery of wikimedia commons,Author haade
Dalton, was the first one in publishing a table of atomic relative weight. Six elements appear in this table: hydrogen, oxygen, nitrogen, carbon, sulfur and phosphorus, attributing convencionalmente to the atom of hydrogen the weight of a unit.
atomic - molecular Hypothesis of Dalton.
In 1803, it exhibited his atomic theory – molecular, that was explaining and of base to his philosophy, where the laws consider them with three hypotheses.
1-. The elements are formed by atoms, all the atoms of the same elements are equal, between if and different from atom of other element, in such a way that Dalton did not know the existence of the isotopes, that is to say, that the atoms of the same element can be anything different.
2-. Them composed they are formed by molecules of the same compound, are equal between if and different from the molecules other compounds; the molecules it contains the atoms, which form the compound.
3-. The chemical combinations constitute a reordering of the atoms, which were forming the molecules of the initial substances, which gather together forming the products of the reaction.
Thanks to Dalton, a new philosophical base took under the scheme of the chemistry, of such a way, that the analysis and the synthesis limit to the separation or combination of the particles, respectively, no new creation or destruction of the matter is within reach of the chemistry.
Also we have other contributions, which gave the compression to phenomenon of temperature, since it it was of describing thanks to his experiments, to determine the pressure of water steam, in several points between 0 and 100 °C (32 and 212 °F), Dalton came to the conclusion from the remarks of the steam pressure of six different liquids, that the change of the steam pressure, for all the liquids it is equivalent, for the same change of the temperature, determined from steam to any pressure.
scientific East was enduring a less common type of blindness to the color, the deuteranopia, in which the cones sensitive to medium wavelengths are absent, instead of working with a form mutada of his pigment, as in the most common type of blindness to the color, which today for study of the medicine is the Color blindness, so much is the case, which donated his eye after his death, so that it was serving as study on what he was suffering, and they will advance more the study of this pathology.
Bibliography.
Essentials of chemistry - Page 90 for Ralph A. Burns - 2003.
Chemistry 2: Calculations in the reactions and chemistry of the carbon for Víctor Manuel Ramírez Regalado - 2015.
Introduction to the history of the chemistry for Soledad ESTEBAN SANTOS - 2010.
