Supercooling Water and Black Ice

Welcome dear readers to another fascinating phenomenon I would like to show you. Today’s article is about supercooled water and black ice. Furthermore I will briefly address to superheating as well. Before we start I want you to have a look at the following videos. In the first video we see water poured into a bowl and as soon as the water hits the bowl it becomes ice. In the second video something is put into the water and it magically turns into ice too.
Fig 1. First experiment with supercooled water Credits
Fig 2. Second experiment with supercooled water Credits
In yesterdays post about hot water freezing faster than cold water we learned about the intermolecular forces that are responsible for the actual state of matter. These intermolecular forces are directly related to the pressure and temperature. The higher the pressure the nearer the molecules and therefore higher the intermolecular forces. The following diagram is called a phase diagram and describes how many phases a substance has and in what state of matter it is.
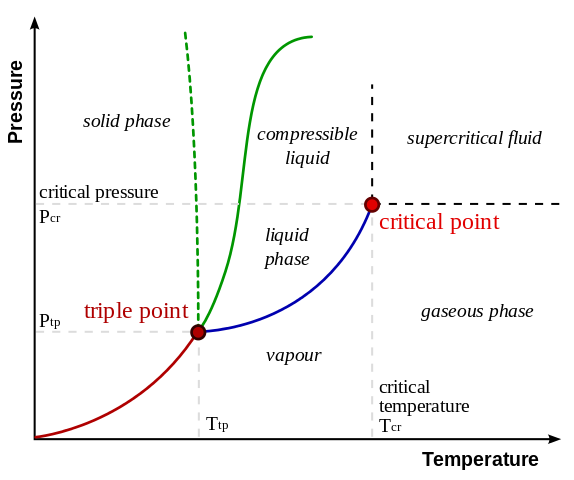
Fig 3. Phase diagram of common substances and water(dashed line) Credits
This pressure-temperature diagram helps us to understand under what condition a substance changes its state of matter. First of all we have two red dots here: the triple point and the critical point. The triple point describes the condition where all three phases can exist. Means we have liquid, solid and gas at this point. The lines show us the phase boundary. If we move from the triple point to the critical point (blue line), we are moving on the phase boundary between the gaseous and liquid phase. The line stops at the critical point and doesn’t reach further. This has the reason that above the critical temperature and critical pressure, liquids and gases have the same density. It is not possible to further distinguish between a gas or liquid above the critical point, thus we have no phase boundary above this point. This state is called supercritical. To give you a little example look at the red line, the sublimation line, which starts at the point of origin and goes to the triple point. Below the triple point pressure (Ptp) a substance can only be solid or gaseous. Is the temperature below the triple point temperature (Ttp) the phase is solid. Above this temperature we have a gaseous phase. Right on the line we have both phases which merge into each other. The dashed line shows the anomaly of water. Water has its highest density and lowest volume at 3.98°C. Above this temperature the density declines and at 0°C the the volume increases.
Supercooled water
The freezing point of water is at 0°C. Water usually turns into a solid at temperatures below 0°C. Under certain conditions however, water can still remain as a liquid below 0°C. How is that possible?
In order to freeze, a liquid needs seed crystal. This can be for instance a germ, dirt, dust etc. (any particle). Ice crystals can only grow on other crystals. In the absence of such seed crystal the water can’t turn into a solid (ice). If we have pure water the water can cool down to -70°C without freezing. Now comes the interesting fact. In the video we saw that the water turns into ice once it’s poured or something is put inside. He puts a seed crystal to the water and it instantly turns into a solid. The second way is through kinetic energy. If you hit the bottle or pour it, it can turn to ice as well. Usually the water molecules have an arranged structure, when hit however, they build a kind of cluster where the crystallization can happen. Because undercooled water is so sensitive it is called a metastable state.

Fig 4. Spontaneous crystallization Credits
Undercooling is happening in nature as well. Raindrops can cool to under 0° and fall to the earth. If these raindrops fall through very clean air layers they don’t turn into snow and reach the ground as a liquid. In the moment they hit the surface they turn into ice. This is also known as black ice.

Fig 5. Black ice on a street. Visbily by the mirror-like reflection Credits
Superheating
Just like supercooling, water can superheat. The process behind is basically the same. In order to boil, water needs a seed crystal. Usually water evaporates at 100°C. In the absence of a seed crystal water can reach a temperature of even 110°C or above without evaporating. This is very dangerous! As soon as you put something to the water or shake the container, the hot water spreads explosively and can hurt you badly. Working in a laboratory we have to take precautions to avoid such risk. Superheating often happens in long and narrow vessels that have a smooth surface. To avoid the splashes you can carefully stir the liquid or put a seed crystal before heating.

Fig 6. Sudden evaporationCredits
Don’t be afraid the next time you cook. Your cooking pot has a rough surface inside to prevent superheating.
Thanks for reading and following me! Be sure to check out tomorrow's article!
Yours,
Tim
Interessing to know!
Thank you my friend
It is simple physics, but still very impressive, informative and interesting, thank you.
I totally agree. Science!
what a nice experiment. 대단하네요^^
Thank you. What does the last string mean? What language is that?
It's Korean. It means you're great. Haha.
I think pretty cool as well
Great stuff, resteemed!
Thank you!
Pretty neat stuff.
Thank you bola! :)
Nice post here Tim. Well done.
Thank you for the feedback!
Very cool if you forgive the pun.
Haha, actually the best comment