Creating instant ice – What is supercooled water?
Supercooling is the process of chilling a liquid below its freezing point, without it becoming solid.
In the experiment I conducted (shown below), I placed the water in a freezer for around an hour. However, when taking the water out of the freezer it was not yet frozen. As can be seen from the video, when I banged the water on the table it began to freeze. Seems strange, right? How could the water possibly remain liquid if it is below its freezing point?
Why does the water not freeze when put in the freezer?
At room temperature, water is a liquid. It remains in this state of matter because all the water molecules have relatively high kinetic energy. They are able to vibrate and move past each other. This is what forms the basis of a liquid state of matter. Once the water is placed in the freezer it beings to cool, and the kinetic energy of the molecules decreases. Eventually the molecules should align and become relatively fixed through their hydrogen bonding to form solid ice. This is shown in figure 2 below.
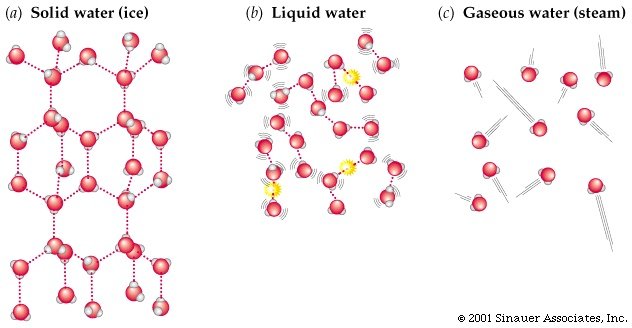
figure 2. Solid, liquid and gas
Ice crystals will form easily when growing on other ice crystals. But, in order to initiate this crystallisation event there must be a nucleation site in the water. This nucleation site is just a place within the water that enables crystallisation to occur. Initially, a small number of ions, atoms or molecules will become arranged in the typical pattern of the crystalline solid. This also becomes a place where the crystal grows from. If the water is not pure, then the impurities within it can act as sites of nucleation to begin the crystallisation process. Without these impurities in the water it is very difficult for the water molecules to find a nucleation site to allow the ice to form. It is therefore important that pure water is used in this experiment, or the water will simply freeze when left in the freezer. When the water is in this supercooled state (a liquid below its freezing temperature) it is said to be metastable.
Why does the water change to ice when banged on the table?
By rapidly shaking or banging the water bottle on the table this will disrupt the water molecules within it. By doing so it will cause some of the molecules to align in a specific way to trigger a nucleation event. Ice will then grow from that specific point, as seen in the video.

figure 3. Demonstration of supercooled water
In figure 3 an ice cube itself is used as a nucleation site.
Why should we care?
Apart from looking pretty cool, what is the point of conducting this experiment, or knowing the chemistry behind supercooling?
Strangely enough, supercooling chemistry is used to help people stay warm. There are re-usable hand warmers, made of a solution of sodium acetate and water, that employ supercooling to store heat for later use. First the hand warmer is warmed up in boiling water. Upon removal, the sodium acetate cools to room temperature. This is below its freezing point, but like with our water bottle, it remains liquid as there is no site for a nucleation event to occur. By bending a metal disk inside the warmer, a nucleation site is created and crystals can form in an exothermic reaction, giving off heat.
Supercooling is also being tested for use in organ preservation for transplants. By supercooling livers, they were able to be preserved for 96 hours, which is four times longer than the standard preservation procedure.
This is experiment can easily be done in your home if the correct water is used. From my experience the best water to use is Fiji water, and can be purchased in most supermarkets. However, it may take several attempts to be able to supercool the water in correctly.
References:
How to supercool water
Supercooled water
Supercooling applications

Is it just me? I think this post is SUPERCOOL! Haha. A very good read bro. Thanks for the knowledge :D
No problem, hopefully many more to come.
Its also possible to replicate the 'supercooling' effect with other mixtures of liquid - for example beer, (which although I have done myself, this video from mythbusters is a better shot concept) which whilst it might typically contain about 4-5% alcohol by volume, plus dissolved carbon dioxide and certain other compounds can still be chilled to a point below its regular/expected freezing point & instantly frozen. It's a little trickier to get right though as it needs to be slightly colder than water alone due to the alcohol and other compounds present. Makes a neat party trick though!
I agree.
This post is wayyyyy too cool!!! I understand supercooled water droplets as a pilot, but DANG! This was seriously a great post! Well done @ovij :)
Awesome post!! man that is badass i frikin love science
Thanks, glad you enjoyed it!
This was awsome. Ill try this at home. What temperature do i have to hit to make the reaction possible?
SteemON!
@reported has voted on behalf of @minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond. To be Resteemed to 4k+ followers and upvoted heavier send 0.25SBD to @minnowpond with your posts url as the memo
I'm surprised supercooling a liver would work - seems like crystals would form somewhere in a liver - I wonder how they prevent that from happening?
They used a specialised solution to protect the liver sinusoids and epithelial cells from the potential damage of freezing and ice crystals. Polyethylene glycol (PEG) was used to protect the epithelial cells and a glucose derivative 3-O-methyl-D-glucose (3-OMG) was used to protect from supercooling.
More information can be found from the study here:
http://www.nature.com/nm/journal/v20/n7/full/nm.3588.html
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
I should try this too :D
I have seen this a while ago but i forgot, it's cool!
Thanks for this. Does that mean that the freezing point for clean water is below zero Celsius? I'm resteeming and upvoting this. 😀 😃 😄 😁 😆 Thanks again for sharing...and don't forget to keep steeming OK?
very nice blog. really full of information.