So just how did this chicken get frozen?!?!
Refrigeration is everywhere. In your home, in your local supermarket, in the pub, in restaurants, in server rooms, you name it and there is probably some form of cooling that is required. Before I started in the refrigeration industry I never thought twice about it, into what it actually takes to freeze a chicken or chill a beer. It is a fascinating science, and one that keeps the civilised world, well, civilised. Without it we would not be able to keep our perishable foods for more than a day or two, disease would spread like wildfire and no beer anywhere would be nearly as satisfying as it is now.
The idea of preserving food dates back to the Chinese and Roman empires, but mechanical refrigeration has seen massive development in the last century or so. In the U.S the introduction of refrigerated rail cars led to westward expansion in areas that were previously not on main transport routes. The result was growth into major cities, in places always thought to be inhospitable, like Las Vegas, Nevada and Houston, Texas.
The earliest forms of cooling were done in the form of ice harvesting, since the time of the Persian Empire, with historians saying that this was mainly done to chill beverages. The Jews were another ancient civilisation to harvest ice, with the book of Proverbs reading “As the cold of snow in the time of harvest, so is a faithful messenger to them who sent him.” By the 1830’s, ice had become a mass-market commodity, with about 100,000 tons being used in New York City in 1856. This created a culture of cooling, with people storing ice in insulated ice boxes and ice houses, to preserve dairy and meat products.
Today however, I believe the world would completely fall apart if all refrigeration suddenly had to come to a standstill. It is something we see all around us every day, but seldom take the time to think of how it all works, how it is possible to keep something at -20°C in a room when outside that room the ambient temperature could be well above 40°C.
The biggest misconception of refrigeration is that you are creating cold. This is completely untrue. What refrigeration actually does is remove heat. ‘Cold’ is not something that can be created, it is merely the absence of heat. The most fundamental way I can explain how a refrigerator works is as follows:
Imagine you have a bucket filled with water (the bucket being a room or area and the water being heat). Now you have a second bucket that is empty (representing the area outside the room), and between the two is a sponge (representing the refrigerant gas). You take the sponge and put it in the bucket containing the water (heat) and allow it to absorb. Then move the sponge to the empty bucket and squeeze the absorbed water into there. Continue this process until the ‘room’ bucket is empty and the ‘outside’ bucket is full. Result: all heat removed from the room and rejected to the outside ambient air!
But how does a real refrigeration system do this? Let’s start with a parts breakdown (just the main components), I will compare them to parts of the human body:
- Compressor (the heart of the system)
- Tubing (the veins)
- Refrigerant gas (blood)
- Evaporator (inhaling lungs)
- Condenser (exhaling lungs)
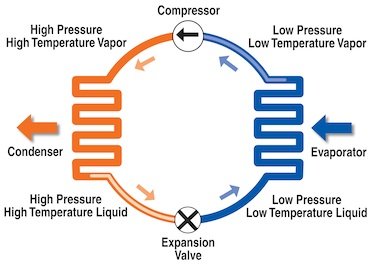
The system is split into two sides, the high pressure side and the low pressure side. The cycle starts at the compressor. The compressor does exactly that, compresses the refrigerant into a high pressure, high temperature vapour. This vapour then flows to the condenser via the tubing and gets condensed into a high pressure, high temperature liquid. At this point any excessive heat has been rejected to the outside air, being blown away by a fan fitted to the condenser (imagine an air-conditioning outdoor unit, this is simply a condenser with its fan blowing off the heat). The liquid then flows from the exit of the condenser to an expansion device, which is either in the form of an expansion valve or a capillary tube. This is where Boyles law comes into play, where there is a drop in pressure there is drop in temperature. The liquid is ‘sprayed’ from the expansion device into the evaporator. We are now in the low pressure side of the system. Have you ever noticed that aerosol deodorant spray is always cold when you spray it onto your body? The exact same thing is happening here, however the refrigerant’s chemical makeup makes it ideal for this application. Once in the evaporator (which is inside the room or cabinet), the refrigerant is a low pressure low, temperature liquid and it begins absorbing the heat, via the fins and coils of the evaporator. As it absorbs more heat, the refrigerant begins to boil and evaporate. The refrigerant is actually boiling inside there, even though it might be at -30°C! The refrigerant is now in the form of a low pressure, low temperature vapour, carrying heat that has been absorbed from the air moving over the coil. As the compressor continues to run, the vapour is pulled back to the compressor, now carrying any heat absorbed with it. It is again compressed and condensed, with the excess heat being rejected to the outside air. This cycle continues until the room is at the set temperature, where a device like a thermostat will register that the desired temperature has been reached and switch off the compressor. As temperature rises in the room again, normally a differential of about 2°C, the thermostat registers this and switches the compressor back on again and so the cycle continues.
This principle remains true for just about any refrigeration or air-conditioning system you can think of. From your domestic fridge up to huge multiplex systems that run entire supermarkets from a central plant. Think back to the bucket and sponge scenario and read through the above again, and you will see the basic principles in play.
I hope this has been informative, it truly is a fascinating science, albeit that the fundamentals are very basic. So next time you pick up that ice-cold beer or have a scoop of ice cream, remember the science that has gone into getting it to the perfect temperature!
If you enjoyed this (my third blog!) please share and follow me @ollie7!
Sources:
www.wikipedia.org/wiki/refrigeration