Comparing (eco)toxicity of commercial and home-made laundry detergents... as expected, results are appalling...
I have worked as a research chemist for many years in a water treatment company. The knowledge I acquired during that period of my life allows me to be appalled when I read the chemical composition of some commercial or industrial products. The thing is, alternatives do exist. The reason why these are not implanted on an industrial scale, I’ll let you guess…
What I will do here is follow up on an inspiring article I read a few days ago on Steemit. It was written by @delph-in-holland:
https://steemit.com/health/@delph-in-holland/my-homemade-laundry-powder-recipe.

Delphine presents an alternative recipe to laundry detergents. She developed it herself by trial and error, and has been using it now for years with success.
In her article though, she admits lacking certain knowledge in water chemistry, which implies that she couldn’t propose strong scientific arguments on why it should be used instead of what is proposed in supermarkets.
This is where I jump in.
This article will compare the toxicity and eco-toxicity of Delphine's eco-aware laundry powder recipe with that of a commercial laundry powder you can buy in supermarkets.

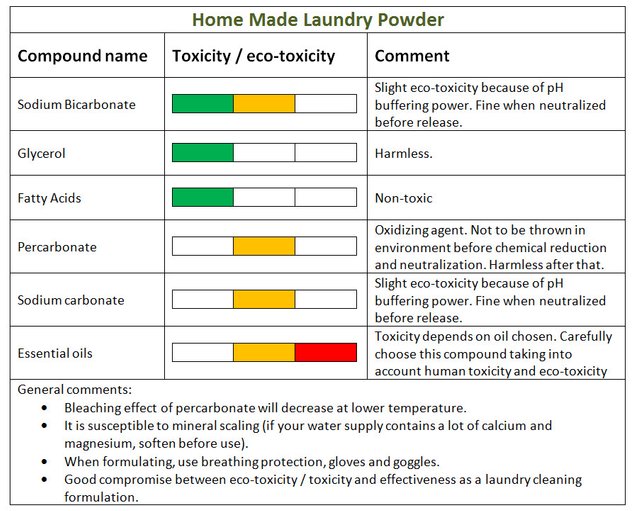
Delphine's home-made formula
Let’s start with Delphine's home-made recipe and discuss the ingredients.

Marseille soap is made by saponification. Saponification is a well-known reaction that has been used for millennia in order to produce soap:
Fatty esters (triglycerides) + caustic soda --> glycerol + sodium salt of fatty acids.
Glycerol is not toxic neither for humans [source], nor the environment [source]. It is actually edible.
Fatty acids easily biodegrade in the environment and are non toxic for animals and humans [Source].
Sodium carbonate (Na2CO3) should be handled with care by humans. It can provoke strong irritations upon contact and inhalation. Consequently, use gloves, eye protection and a mask when manipulating large quantities [Source]. Sodium carbonate is not toxic or ecotoxic per se, it is its buffering pH that can cause a problem if released in the environment without care. It should be neutralized first which is done easily in water treatment plants.
Sodium bicarbonate (NaHCO3) is non-toxic and non-ecotoxic. As with sodium carbonate, bicarbonate has a strong pH buffering power (that’s part of why it's effective), it should not be released in the environment without prior neutralization.
Percarbonate is actually sodium carbonate bounded with peroxide. Peroxide is an oxidant so it is harmful to life, however, when released, it degrades into sodium carbonate, and peroxide which turns into oxygen and water. [Source]. During such degradation, molecules surrounding the peroxide can be oxidized, but this process can occur in the sewer and at a water treatment plant. So when handled correctly, it is eco-friendly. In the home-made formulation, Percarbonate takes the role of the bleaching agent.
Essential oils are used to perfume the laundry powder. They do not intervene in the cleaning process, although they can act against bacteria and fungi, protecting clothes. That’s a good thing, right? Well, this is actually also the problem… many essential oils are eco-toxic, but the concentration being very low it may be acceptable to use them in certain conditions.
By definition, essential oils contain aromatic compounds and should be treated with care. Some can be very toxic, even carcinogenic: You shouldn’t dump any essential oil in your laundry just because it smells good. Check the toxicity and ecotoxicity of the oil or oils you wish to use first.
The step by step procedure to formulate your own laundry powder is provided on Delphine's post. Please check it out (and upvote her post): https://steemit.com/health/@delph-in-holland/my-homemade-laundry-powder-recipe

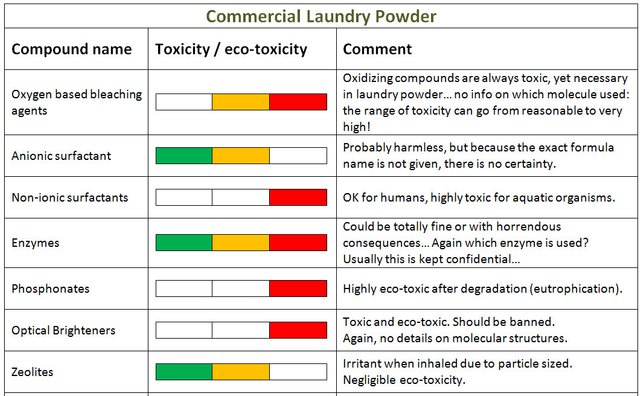
A typical commercial formula
Now let's discuss the ingredients of a typical laundry powder found in supermarkets:

Oxygen based bleaching agents… Well the problem here, is that we do not really know what these are… if they are percarbonate-based, it is not better or worse than the home made product. On the other hand percarbonates have limited action below 60C, so industrials tend to try and boost effectiveness by adding chlorine based products (bleach derivatives), and the aqua toxicity of the degradation products which are chlorinated is just a nightmare.
Perborate is sometimes used also, although not as bad as chlorinated products, it is less eco-friendly than percarbonate. In addition it requires an activator, tetraacetylethylenediamine. The name might scare you but this latter compound is actually OK [source].
For anionic surfactants, well, it is difficult to say here too: the molecular structure is not given. In the best case scenario these are standard fatty acids used in home-made soaps.
Non ionic surfactant: In hard waters (containing Calcium for example), standard surfactants can be deactivated. This is why non-ionic surfactants are added. These often take the form of alcohol ethoxylate, which can be highly toxic to aquatic organisms. Again, the fact that the exact formulas are not divulged in their entirety is a scandal, limit criminal.
Phosphonate: Extremely eco-toxic. Phosphonates will degrade readily into ortho-phosphate which is food for green algae. Phosphate released in the environment ends up giving a huge competitive advantage to green algae versus other species. The algae soon covers water surfaces and by doing so, cuts the light from the sun to all life located underneath the surface. This phenomena is called eutrophication and can destroy thriving aquatic ecosystems.

Zeolites are not chemically toxic: it is the size of the zeolite particles that can cause health issues not their chemical composition. So naturally, when added to water they lose their toxicity. Their roles in laundry powders is to soften the water (remove calcium and magnesium ions that deactivate the ionic surfactants).
Enzymes: These have a beneficial effect in breaking molecules making the stains on clothes. Again the problem here is the non-divulgation of the exact formula. Some enzymes degrade well at relatively low temperatures and are harmless for the environment, some have high risk of spreading out and create large problems for the environment and are toxic…
Which one is used in a specific powder? Not always easy to find out for the basic consumer... Do we trust chemical corporation enough to have carried out an objective and fully funded toxicological and eco-toxicological study? I don’t. I have to remind myself that the primary focus here for these companies is to make profit only...
Optical brighteners: These toxic and eco-toxic compounds are used to make your clothes appear brighter (not cleaner, brighter…). Everything is said in this resource. And again, there is no mention of the detailed molecule used. In any case, use of this additive is just plain bad.
Perfumes: Same here, no info on which molecule is used. Some are perfectly fine, some can be really toxic… We just have to trust the food agencies and the companies. No f****g way!
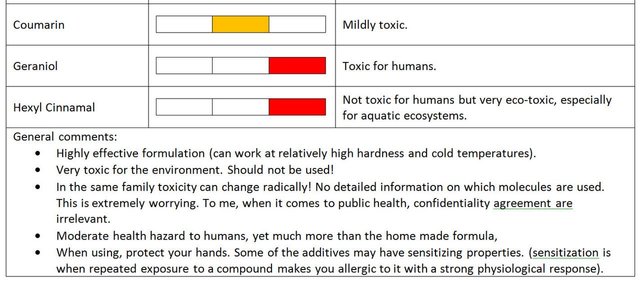
Coumarin is a mildly toxic vanilla flagrance. It has been banned in some countries as food additive [source].
Geraniol is a flagrance with recognized toxicity. Download link of MSDS (material safety data sheet):
Hexyl Cinnamal is not really toxic for human but very toxic for aquatic life. Download link of MSDS
Polycarboxilate are dispersing polymers that counter water hardness and can synergistically improve the effectiveness of phosphonates as corrosion inhibitor. So in other words, polycarboxilates improve the action of ionic surfactants by dispersing calcium and magnesium in the water, and can help in preventing corrosion. (source: my own research - published internally).
For standard compounds like polyacrylates, toxicity and ecotoxicity is negligible, but again, only the compound's family name is provided. So in the end, behind a sweet smile one could find a substituted polycarboxilate with active functions that could render it seriously ecotoxic.
Now Let's Compare !
and

Conclusion
So, after reading this text or/and consulting the references provided, what do you think?
In my opinion, we are allowed to breathe even if our bodies release green house gases like CO2. A home-made laundry powder is far from ideal, but we as a species, have the right to wash our clothes, in order to keep a good hygiene and minimizing the risk of disease. But to keep that right, we have to make wiser choices in terms of environmental sustainability. Delphine's formula shows us that alternative solutions to eco-toxic industrial products do exist.
What we can also pick up from this study is that standard laundry powder found in all supermarkets are eco-toxic, aqua-toxic and sometimes even harmful to humans. Worse of all, the lack of clarity about the individual components is very worrying. Are industrials hiding behind the low toxicity averages of a whole family of compounds?
If you do not feel like preparing your own laundry yourself, there are alternatives that you can purchase on the internet. Armed with what you learned here, you should be able to make the right choices.
As a starting point, check http://grist.org/article/its-a-wash/
Now, it’s up to you… make the right choices for our future !

Illustration credits:
Background of initial picture : Dr. Jennifer L. Graham | U.S. Geological Survey
Elements used to build frieze and images: Pixabay.com

Hi,
I’m @muphy, My life revolves around music production, teaching sciences, and discovery through travel.
You enjoyed that post? Resteem and Upvote!
You are interested in these topics? Follow me!
I just forwarded your post to my wife who is taking care of making our own laundry power. I can bet that she will be really happy when she will read your post. This also strengthens our motivation to move away from any commercial laundry powder at all.
(Her recipe is even simpler than the one of @delph-in-holland, but I will let her comment on this.)
Thank you @Lemouth.
I did the scientific analysis of the situation by comparing the toxicity of the two formulas in order to provide arguments on why an alternative formula like Delphine's is much better for the planet! It was fun to dig back into an old area of expertise just for one day :-)!
Delphine did the empirical research and expressed her years long experience in her article by sharing her formula. Your wife has gone on the same path, ending up with her own formula.
I am pretty sure that both of them would enjoy exchanging tips and tricks. She is French too and digs into various areas to look for sustainable solutions. Putting their experiences in common, Who knows, they might even come up with The Optimal Formula :-)!
We actually started to move away from the industry for the reason we have not control on what is in the powders (same holds for soap, skin creams, etc...). We are not against the industry, but we want to know what the products we use everyday are made of. And today, it is sad to say that the only way is thus to make everything by ourselves. We are far from there, but getting closer and closer ;)
Lol :)
"We are not against the industry, but we want to know what the products we use everyday are made of. "
That is exactly the point!
Industrialization is related to demography, and needed for a dense society to develop.
But today, trust is gone...
I just read your post that I found really interesting. I will also read that of Delphine to learn more about his own homemade laundry recipe. For my part, I wanted to try this experiment for several reasons (including the one mentioned by @lemouth). Another one he didn't mention is the extremely fragile skin of our second son and my categorical refusal to put him to corticoids ...
As for my recipe, it is really very basic: water, Marseille soap, soda crystals and vinegar. I do not yet have the necessary perspective to judge the effectiveness of this recipe since I'm at my first experience in this area! ;)
Yes, it is a very wise choice to not use industrial detergents when washing the clothes of your second son. For example, by personal experience, polyphosphates and pyrophosphate can have quite aggressive on the skin, and some additives that can even be sensitizers (exposure can make a person become very allergic to it in the future, even when exposed to extremely minute dose of the additive) !
The two formulas are similar actually. But Delphine (@delph-in-holland) is the expert on the practical effects, I'll let her comment :-)
Please, let us know how happy you are with the results of your first formula !
I will surely write some post on the subject in the future ;) Your expertise had been very helpful to understand "why" it was better like this! Thanks to you!
@lemouth, I am curious to know your wife's recipe!
She is @lamouthe on steemit. I forwarded her both your link and the one of @muphy. But we are Dec 30th... Don't expect an answer before a few days ;)
Great post! I’m always interested in eco friendly detergents. I use the grey water in the garden. I make sure not to pour it directly on the plants, just around the base.
Thank you.
Humm, I wouldn't use too much of grey water for your garden, and even if you use basic eco-soap: It is not because a detergent is eco-friendly that it does not have chemical properties while it is active... (Being eco-friendly means that a compound degrades readily and the degradation products are harmless for nature).
I have a garden too , and will probably get active on this with the return of warmer days. You should also follow my friend Delphine (@Delph-in-holland), she is a gardener and very much into developing and using nature-friendly solutions to gardener problems!
For example, we rent a larger common garden together, and we are testing / using permaculture techniques.
thank you for the reply, i will take your advice. I will start to follow her as well. i enjoy gardening posts!
Great article, I enjoyed the perspective of looking at the chemistry!
One of my favorite substances to use is the old timer's cleaning agent: Borax.
A 2001 study found that an estimated 10% of the population has their health adversely affected by liquid laundry detergents, especially as the volatile gasses out-gas in heated driers ( https://www.sciencedaily.com/releases/2011/08/110824091537.htm ).
To make maters worse, as far as I know, there is no regulation that states that these companies have to list everything that they use on the label. Therefore, one really has no way of knowing exactly what is in these chemical detergents.
I just recently switched to natural deodorant, and we've used natural detergents for quite some time.
Super comment @jzen, thank you.
Well, be careful though. It is not because a product is 'natural' that it is not toxic or eco-toxic. For example, I use Alun stones as deodorant under arm pits. But I do it very seldomly (a couple of times a month). Although it is natural, it contains al3+ salts which are carcinogenic!
I also notice that since I stopped using deodorant, I do not need it, and I don't stink!
For all alternatives you wish to use (like borate), Look for and Check the MSDS (material Safety Data Sheet).
For example Borate tetrahydrate (Borax) appears OK, but its acid version is pretty crap... So be careful.
Borate: http://www.sciencelab.com/msds.php?msdsId=9924967
Boric Acid: http://www.sciencelab.com/msds.php?msdsId=9927105
Check sections 11 and 12 for more detailed toxicological info.
Thank you all for your upvotes and interest in this article.
If you enjoyed it and, most importantly, believe that such info should be widely spread, please have a look and upvote the article that inspired me to dig deeper:
https://steemit.com/health/@delph-in-holland/my-homemade-laundry-powder-recipe
There has never been a better time to take control of your clothes by using a sustainable, all natural laundry detergent. Whether you choose to make your own detergent or experiment with soap nuts is up to you. In both cases, you will be benefiting both your body and the environment by reducing exposure to toxic chemicals and by keeping the ocean and landfills free of unnecessary plastics. So wash away!
This is well detailed sir, i would have to try making the alternative.
excellent post friend congratulations you have my vote
Great post there, keep up good work !
This replay was created using STEEMER.NET Alpha ( support STEEMER.NET Transactor / Wallet / Exchange Project here: https://steemit.com/investors-group/@cryptomonitor/steemer-net-steem-blockchain-transactor-for-windows-android-app-funding-update-243-1200-sbd-28-12-2017 )
💙 Great post. Very good writing,i like this post 💙
Congratulations @muphy! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP